

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM31490 substituted biphenyl derivative, 36ad
SMILES: CC(C)CNC(=O)c1ccc(c(c1)C(O)=O)-c1ccc(cc1C(=O)Nc1ccc(cc1)C(N)=N)-c1ccc[nH]1
InChI Key: InChIKey=XTUJNPODMRCIHC-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII (Homo sapiens (Human)) | BDBM31490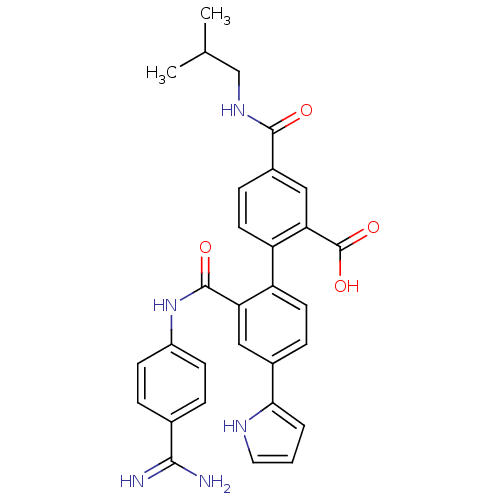 (substituted biphenyl derivative, 36ad) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals | Assay Description TF-FVIIa assay reactions were performed in a mixture containing FVIIa , lapidated tissue factor, in an assay buffer in the presence of test compounds... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM31490 (substituted biphenyl derivative, 36ad) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals | Assay Description FXa assay reactions were performed in a reaction mixture containing FXa, and test compounds were added at varied concentrations and reactions were in... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM31490 (substituted biphenyl derivative, 36ad) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals | Assay Description Thrombin assay reactions were performed in a reaction mixture containing thrombin, and test compounds were added at varied concentrations. The reacti... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM31490 (substituted biphenyl derivative, 36ad) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | 25 |
BioCryst Pharmaceuticals | Assay Description Trypsin assay reactions were performed in a mixture containing trypsin, and test compounds were added at varied concentrations. The reactions were in... | Bioorg Med Chem 17: 3934-58 (2009) Article DOI: 10.1016/j.bmc.2009.04.013 BindingDB Entry DOI: 10.7270/Q2SX6BJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||