

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NCCC(O)c1cccc(OCC2(O)CCCCC2)c1
InChI Key: InChIKey=LOMJAUAGGZEKMG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoid isomerohydrolase (Homo sapiens (Human)) | BDBM323446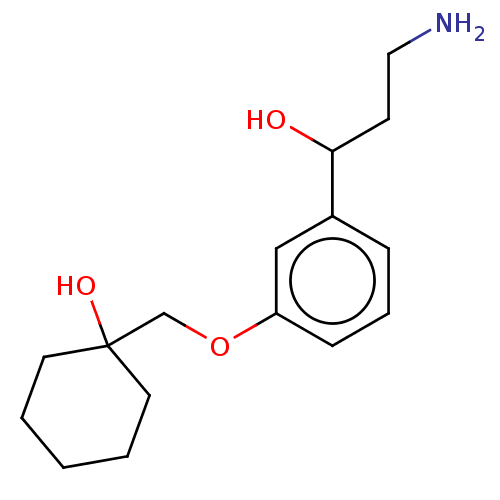 (1-((3-(3-Amino-1-Hydroxypropyl)Phenoxy)Methyl)Cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ACUCELA INC. US Patent | Assay Description Isomerase inhibition reactions were performed essentially as described (Stecher et al., J. Biol. Chem. 274:8577-85 (1999); see also Golczak et al., P... | US Patent US10188615 (2019) BindingDB Entry DOI: 10.7270/Q2NP26HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinaldehyde-binding protein 1 (Homo sapiens (Human)) | BDBM323446 (1-((3-(3-Amino-1-Hydroxypropyl)Phenoxy)Methyl)Cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
ACUCELA INC. US Patent | Assay Description Isomerase inhibition reactions were performed essentially as described (Stecher et al., J Biol. Chem. 274:8577-85 (1999). | US Patent US10639286 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinaldehyde-binding protein 1 (Bovine) | BDBM323446 (1-((3-(3-Amino-1-Hydroxypropyl)Phenoxy)Methyl)Cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
ACUCELA INC. US Patent | Assay Description Isomerase inhibition reactions were performed essentially as described (Stecher et al., J Biol. Chem. 274:8577-85 (1999). | US Patent US10639286 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoid isomerohydrolase (Bos taurus (Bovine)) | BDBM323446 (1-((3-(3-Amino-1-Hydroxypropyl)Phenoxy)Methyl)Cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ACUCELA INC. US Patent | Assay Description Isomerase inhibition reactions were performed essentially as described (Stecher et al., J. Biol. Chem. 274:8577-85 (1999); see also Golczak et al., P... | US Patent US10188615 (2019) BindingDB Entry DOI: 10.7270/Q2NP26HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||