

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM323599 (E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl)phenoxy)-2-methylhex-2-enoic acid ::US10188627, Compound 8k
SMILES: CN(Cc1ccccc1OCCC\C=C(/C)C(O)=O)C(=O)c1ccc(cc1)-c1ccco1
InChI Key: InChIKey=UOUIWYBSJFMAKV-UFWORHAWSA-N
Data: 6 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323599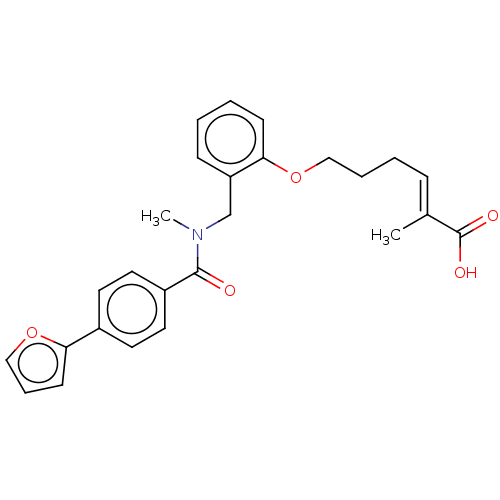 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc.; Salk Institute for Biological Studies US Patent | Assay Description Medium including test compound was aspirated and washed with PBS. 50 μl PBS including 1 mM Mg++ and Ca++ were then added to each well. The lucif... | US Patent US10188627 (2019) BindingDB Entry DOI: 10.7270/Q2HX1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PPAR delta protein (Rattus norvegicus (Rat)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc.; Salk Institute for Biological Studies US Patent | Assay Description Medium including test compound was aspirated and washed with PBS. 50 μl PBS including 1 mM Mg++ and Ca++ were then added to each well. The lucif... | US Patent US10188627 (2019) BindingDB Entry DOI: 10.7270/Q2HX1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARalpha LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARdelta LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Activation of human PPARgamma LBD expressed in CHO-K1 cells by beta galactosidase enzyme fragment complement based fluorescence assay | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM323599 ((E)-6-(2-((4-(furan-2-yl)-N-methylbenzamido)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Mitobridge, Inc. Curated by ChEMBL | Assay Description Transactivation of yeast GAL4 DNA binding domain-fused PPARdelta LBD (unknown origin) expressed in CV-1 cells after 24 hrs by luciferase reporter gen... | Bioorg Med Chem Lett 27: 5230-5234 (2017) Article DOI: 10.1016/j.bmcl.2017.10.037 BindingDB Entry DOI: 10.7270/Q21V5HJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||