

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM329706 1-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-1H- pyrazolo[4,3- b]pyridin-3- yl)piperidine-4- carboxylic acid::US9663522, 1M
SMILES: OC(=O)C1CCN(CC1)c1nn(C(=O)c2c(Cl)cccc2C(F)(F)F)c2cccnc12
InChI Key: InChIKey=CDTBRAGUFMYFOW-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM329706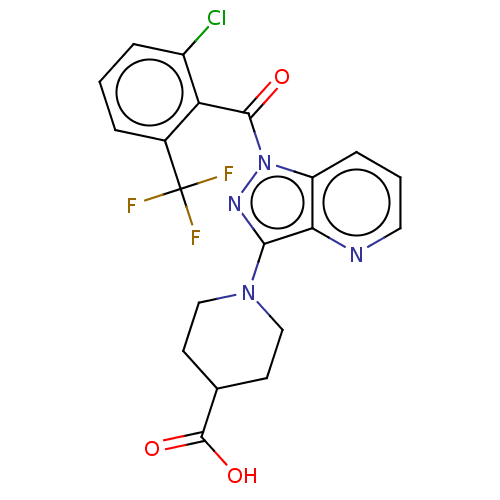 (1-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-1H- py...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 461 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US9663522 (2017) BindingDB Entry DOI: 10.7270/Q2S46V33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM329706 (1-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-1H- py...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 461 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio... | ACS Med Chem Lett 11: 114-119 (2020) Article DOI: 10.1021/acsmedchemlett.9b00431 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM329706 (1-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-1H- py...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Allosteric inhibition of yeast GAL4 DNA domain-fused RORgammat LBD (97 to 518 residues) (unknown origin) expressed in HEK293T cells after 20 to 22 hr... | ACS Med Chem Lett 11: 114-119 (2020) Article DOI: 10.1021/acsmedchemlett.9b00431 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||