 Found 8 hits for monomerid = 344325
Found 8 hits for monomerid = 344325 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM344325
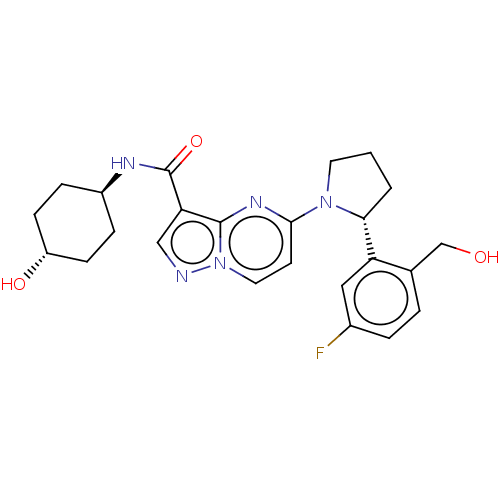
(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
US Patent
| Assay Description
An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... |
US Patent US9782415 (2017)
BindingDB Entry DOI: 10.7270/Q2P84F0G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
US Patent
| Assay Description
Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... |
US Patent US9782415 (2017)
BindingDB Entry DOI: 10.7270/Q2P84F0G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... |
J Med Chem 50: 1876-85 (2007)
BindingDB Entry DOI: 10.7270/Q2R49T22 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
MMDB
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.
US Patent
| Assay Description
Jak2: Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mix... |
US Patent US10758542 (2020)
|
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
US Patent
| Assay Description
Trk enzymatic selectivity was assessed using Omnia™ Kinase Assay reagents from Invitrogen Corp. Enzyme (either TrkA or TrkB from Invitrogen Corp.) an... |
US Patent US9796724 (2017)
BindingDB Entry DOI: 10.7270/Q27M0B21 |
More data for this
Ligand-Target Pair | |
JAK2 (aa 808-1132)
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma, Inc.
US Patent
| Assay Description
Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... |
US Patent US9796724 (2017)
BindingDB Entry DOI: 10.7270/Q27M0B21 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.
US Patent
| Assay Description
An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... |
US Patent US10758542 (2020)
|
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM344325

(US10251889, Example 34 | US10758542, Example 34 | ...)Show SMILES OCc1ccc(F)cc1[C@H]1CCCN1c1ccn2ncc(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:24.25,wD:9.9,27.29,(-6.68,.7,;-7.08,2.19,;-5.99,3.28,;-6.35,4.81,;-5.26,5.9,;-3.77,5.5,;-2.68,6.59,;-3.37,4.01,;-4.46,2.92,;-4.06,1.43,;-4.97,.19,;-4.06,-1.06,;-2.6,-.58,;-2.6,.96,;-1.26,1.73,;-1.26,3.27,;.07,4.04,;1.4,3.27,;2.87,3.74,;3.77,2.5,;2.87,1.25,;3.27,-.24,;2.18,-1.33,;4.75,-.64,;5.15,-2.12,;6.64,-2.52,;7.04,-4.01,;5.95,-5.1,;6.35,-6.59,;4.46,-4.7,;4.06,-3.21,;1.4,1.73,;.07,.96,)| Show InChI InChI=1S/C24H28FN5O3/c25-16-4-3-15(14-31)19(12-16)21-2-1-10-29(21)22-9-11-30-23(28-22)20(13-26-30)24(33)27-17-5-7-18(32)8-6-17/h3-4,9,11-13,17-18,21,31-32H,1-2,5-8,10,14H2,(H,27,33)/t17-,18-,21-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... |
J Med Chem 50: 1876-85 (2007)
BindingDB Entry DOI: 10.7270/Q2R49T22 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data







