

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccc(F)cc1-c1c(F)cnc2[nH]c(cc12)C1=CCN(CC1)[C@H]1CC[C@@H](CC1)C(O)=O
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM354126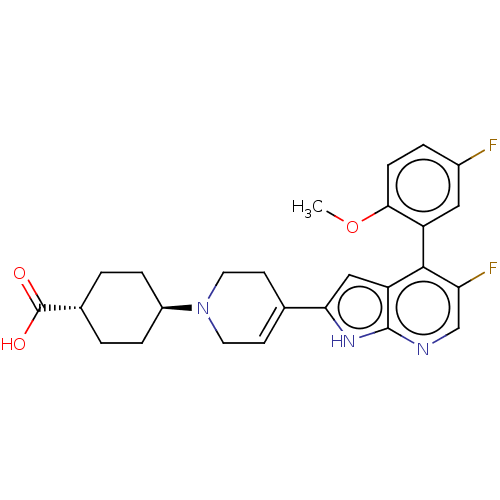 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description CDK9 enzyme activities were measured using LANCE ULight TR-FRET kinase assay reagents (PerkinElmer, Waltham, Mass.). Compounds were directly added in... | US Patent US9796708 (2017) BindingDB Entry DOI: 10.7270/Q2WD42P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK4 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 6 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK6 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9 in human A-431 cells assessed as reduction in RNA polymerase2 phosphorylation at C-terminal ser2 residue incubated for 4 hrs by In... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00161 BindingDB Entry DOI: 10.7270/Q2HM5D8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK7 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 8 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK8 (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9 (unknown origin) using Ulight-labeled substrate measured after 1.5 hrs by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00161 BindingDB Entry DOI: 10.7270/Q2HM5D8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM354126 (US9796708, Example 1358 | trans-4-{4-[5-fluoro-4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK1 (unknown origin) using Ulight-labeled substrate by TR-FRET LANCE method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00744 BindingDB Entry DOI: 10.7270/Q2FB56K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||