

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM363473 (S)-9-(2-(6-(1H-tetrazol-1- yl)pyridin-3-yl)-2-hydroxyethyl)- 4-(4-methyl-5-oxo-2,5- dihydrofuran-3-yl)-1-oxa-4,9- diazaspiro[5.5]undecan-5-one::US9850245, Example 6B
SMILES: CC1=C(COC1=O)N1CCOC2(CCN(C[C@@H](O)c3ccc(nc3)-n3cnnn3)CC2)C1=O
InChI Key: InChIKey=IILZZSPEHVYSAM-QGZVFWFLSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP-regulated potassium channel ROM-K (Homo sapiens (Human)) | BDBM363473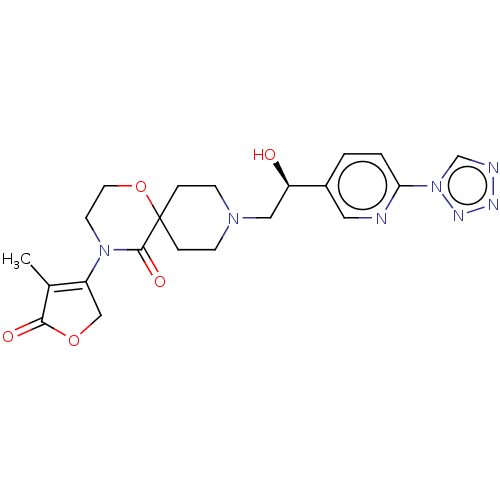 ((S)-9-(2-(6-(1H-tetrazol-1- yl)pyridin-3-yl)-2-hyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Blocking of Kir1.1 (ROMK1) currents was examined by whole cell voltage clamp (Hamill et. al. Pfluegers Archives 391:85-100 (1981)) using the IonWorks... | US Patent US9850245 (2017) BindingDB Entry DOI: 10.7270/Q2H70J3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||