

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM368526 1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-3- yl)isoquinolin-6-amine::US10227343, Example 58
SMILES: FC(F)c1nccc2cc(Nc3n[nH]c4cccnc34)ccc12
InChI Key: InChIKey=RFPRBWBZNYJMKQ-UHFFFAOYSA-N
Data: 1 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM368526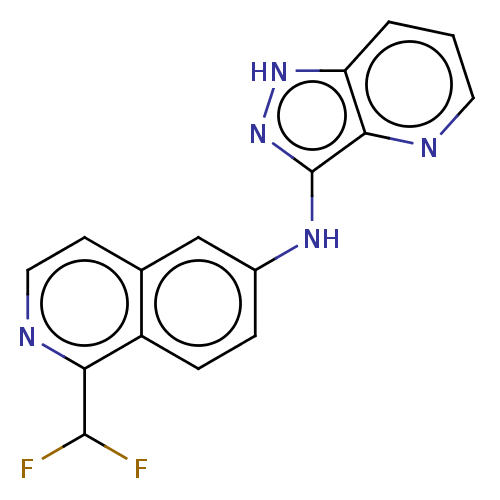 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Universita di Napoli | Assay Description The disclosed compounds and compositions can be evaluated for their ability to act as a potentiator of metabotropic glutamate receptor activity, in p... | J Med Chem 51: 1764-70 (2008) BindingDB Entry DOI: 10.7270/Q2X63Q8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Homo sapiens (Human)) | BDBM368526 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human plasmin using pyroGlu-Phe-Lys-pNA.HCl | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM368526 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human urine urokinase using L-PyroGlu-Gly-Arg-pNA.HCl substrate at 5 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM368526 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human urine urokinase using L-PyroGlu-Gly-Arg-pNA.HCl substrate at 5 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM368526 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human urine urokinase using L-PyroGlu-Gly-Arg-pNA.HCl substrate at 5 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM368526 (1-(difluoromethyl)-N- (1H-pyrazolo [4,3-b]pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of human urine urokinase using L-PyroGlu-Gly-Arg-pNA.HCl substrate at 5 uM | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||