

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM371049 US10239843, Example 156
SMILES: Cc1ncc(Cn2c(=O)n(CC3CC3)c3ccc(cc3c2=O)S(=O)(=O)NC2(C)CC2)s1
InChI Key: InChIKey=VHRYOZHNFLYWFP-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| poly(ADP-ribose) glycohydrolase (PARG) (Homo sapiens (Human)) | BDBM371049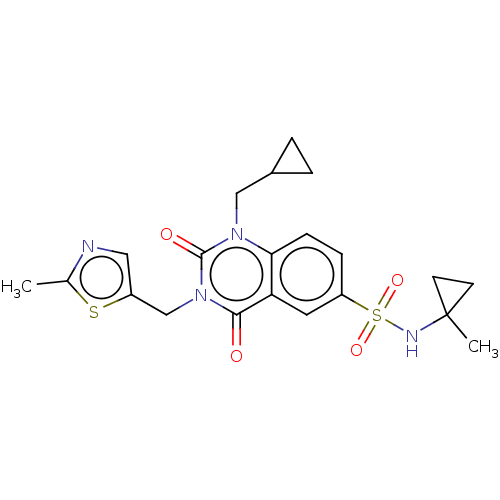 (US10239843, Example 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description PARG In vitro assays were conducted in a total volume of 15 ul in a standard 384 well format. 5 ul of Human Full Length PARG (Produced internally by ... | J Med Chem 51: 1058-62 (2008) BindingDB Entry DOI: 10.7270/Q21R6SSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribose glycohydrolase ARH3 (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description ARH3 In vitro selectivity assays were conducted in a total volume of 15 ul in a standard 384 well format. 5 ul of Human Full Length ARH3 (Enzo Life S... | J Med Chem 51: 1058-62 (2008) BindingDB Entry DOI: 10.7270/Q21R6SSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Superiore di Sanita | Assay Description PARP1 In vitro selectivity assays were conducted as a 10 ul reaction volume in a NUNC Maxisorp 384-well assay plate pre-coated in-house with Histones... | J Med Chem 51: 1058-62 (2008) BindingDB Entry DOI: 10.7270/Q21R6SSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of human PARP1 expressed in Escherichia coli using activated DNA as substrate after 60 mins by peroxy glow reagent A/B based assay | J Med Chem 61: 10767-10792 (2018) Article DOI: 10.1021/acs.jmedchem.8b01407 BindingDB Entry DOI: 10.7270/Q2TM7DTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| poly(ADP-ribose) glycohydrolase (PARG) (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of PARG in human HeLa cells assessed as induction of MMS-induced PAR chains preincubated for 1 hr followed by MMS addition and measured af... | J Med Chem 61: 10767-10792 (2018) Article DOI: 10.1021/acs.jmedchem.8b01407 BindingDB Entry DOI: 10.7270/Q2TM7DTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| poly(ADP-ribose) glycohydrolase (PARG) (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of human full length PARG using Bt-NAD ribosylated PARP1 substrate after 10 mins by TR-FRET assay | J Med Chem 61: 10767-10792 (2018) Article DOI: 10.1021/acs.jmedchem.8b01407 BindingDB Entry DOI: 10.7270/Q2TM7DTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribose glycohydrolase ARH3 (Homo sapiens (Human)) | BDBM371049 (US10239843, Example 156) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of human full length C-terminus His-tagged ARH3 expressed in Escherichia coli using Bt-NAD ribosylated PARP1 substrate after 30 mins by TR... | J Med Chem 61: 10767-10792 (2018) Article DOI: 10.1021/acs.jmedchem.8b01407 BindingDB Entry DOI: 10.7270/Q2TM7DTN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||