

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM373008 US10597366, Compound 163::US9896418, Compound 163
SMILES: CS(=O)(=O)c1ccc(Oc2cc(F)cc(c2)C#N)c2CC(F)(F)[C@@H](O)c12
InChI Key: InChIKey=ONBSHRSJOPSEGS-INIZCTEOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor A (Homo sapiens (Human)) | BDBM373008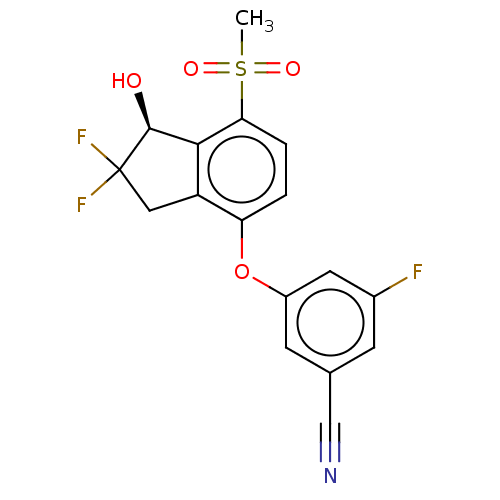 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Research Triangle Institute | Assay Description Four hours later, serial dilutions of 10× compound stocks were made in growth medium from 500×DMSO stocks, and 20 μL of those 10× stocks were ad... | J Med Chem 50: 3686-95 (2007) BindingDB Entry DOI: 10.7270/Q2QC05TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor A (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. US Patent | Assay Description VEGF: Four hours later, serial dilutions of 10x compound stocks were made in growth medium from 500xDMSO stocks, and 20 uL of those 10x stocks were a... | US Patent US10597366 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP2C8 in human liver microsomes using amodiaquine as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP2C19 in human liver microsomes using S-mephenytoin as substrate after 20 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Antagonist activity at HIF-2alpha in human 786-O cells assessed as free plasma adjusted EC50 for reduction in VEGFA concentration after 24 hrs by ELI... | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Antagonist activity at HIF-2alpha in human 786-O cells co-expressing HIF responsive element after 24 hrs by ONE-Glo luciferase reporter gene assay | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Antagonist activity at HIF-2alpha in human 786-O cells assessed as reduction in VEGFA concentration after 24 hrs by ELISA | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged HIF-2alpha PAS-B domain (unknown origin) after 2 hrs by scintillation proximity assay | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP1A2 in human liver microsomes using phenacetin after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP2B6 in human liver microsomes using bupropion as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP2C9 in human liver microsomes using diclofenac as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of of CYP3A4 in human liver microsomes using midazolam as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis | J Med Chem 61: 9691-9721 (2018) Article DOI: 10.1021/acs.jmedchem.8b01196 BindingDB Entry DOI: 10.7270/Q2H997RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of N-(3-Chlorophenyl-4,6-t2)-4-nitrobenzo[c][1,2,5]oxadiazol-5-amine binding to his-tagged HIF-2alpha PAS-B domain (unknown origin) measur... | J Med Chem 62: 6876-6893 (2019) Article DOI: 10.1021/acs.jmedchem.9b00719 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of HIF-2alpha (unknown origin) expressed in human 786-O cells measured after 24 hrs by one-glo luciferase reporter gene assay | J Med Chem 62: 6876-6893 (2019) Article DOI: 10.1021/acs.jmedchem.9b00719 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of HIF-2alpha (unknown origin) expressed in human 786-O cells assessed as reduction in VEGFA level incubated for 20 hrs prior to compound ... | J Med Chem 62: 6876-6893 (2019) Article DOI: 10.1021/acs.jmedchem.9b00719 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. US Patent | Assay Description HIF-2a: The total assay volume was about 100 uL in the following configuration: 2 uL compound in 100% DMSO, 88 uL buffer with protein and probe and 1... | US Patent US10597366 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. US Patent | Assay Description HIF-2a: The total assay volume was about 100 uL in the following configuration: 2 uL compound in 100% DMSO, 88 uL buffer with protein and probe and 1... | US Patent US10597366 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial PAS domain-containing protein 1 (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peloton Therapeutics, Inc. US Patent | Assay Description HIF-2a: The total assay volume was about 100 uL in the following configuration: 2 uL compound in 100% DMSO, 88 uL buffer with protein and probe and 1... | US Patent US10597366 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor A (Homo sapiens (Human)) | BDBM373008 (US10597366, Compound 163 | US9896418, Compound 163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description About 7500 of 786-0 cells in 180 μL of growth medium were seeded into each well of a 96 well plate with white clear bottom on the first day (07-... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q2FN18HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||