

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM38224 US8546380, 1043::US8633188, 1043
SMILES: CC1(CCSC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F
InChI Key: InChIKey=VVZZZUNCWSTIOI-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM38224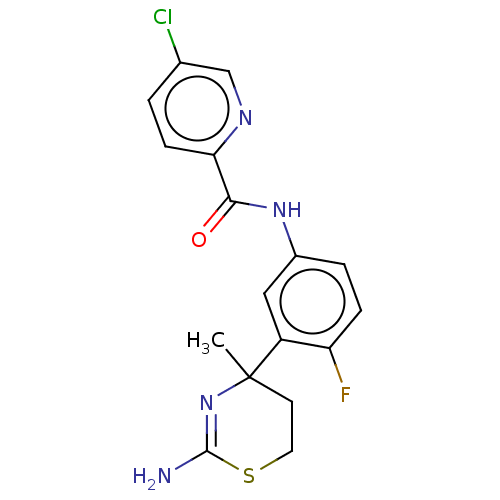 (US8546380, 1043 | US8633188, 1043) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 5.0 | 30 |
Shionogi & Co., Ltd. US Patent | Assay Description Zero point five μL of the test compounds (dissolved in N,N′-dimethylsulfoxide) were incubated with 48.5 μL of the fluorescence-quench... | US Patent US8633188 (2014) BindingDB Entry DOI: 10.7270/Q2FT8JQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM38224 (US8546380, 1043 | US8633188, 1043) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. US Patent | Assay Description Inhibition assay using beta-Secretase activity. | US Patent US8546380 (2013) BindingDB Entry DOI: 10.7270/Q2HQ3XJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||