

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM387827 N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-((1r, 4r)-4-(cyanomethyl)cyclohexyl)-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3-b]pyridin-2-yl)acetamide::US10294226, Compound Ex. 10::US10487083, Example 10::US10981911, Example 55
SMILES: O=C(Cc1nc2cnc3[nH]ccc3c2n1[C@H]1CC[C@H](CC#N)CC1)NC12CCC(CC#N)(CC1)C2
InChI Key:
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387827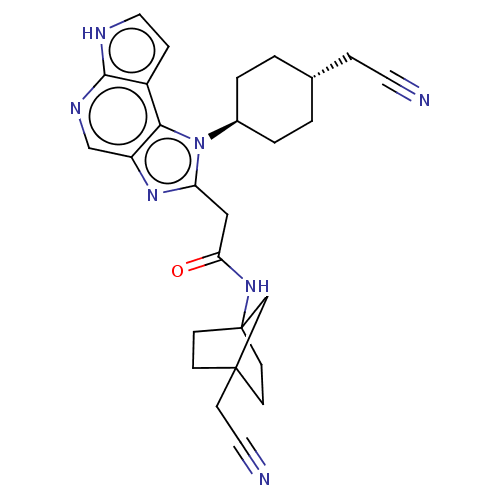 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Substrate (NH2-KGGEEEEYFELVKK-CO2), internal standard peptide (NH2-SWGAIETDKEYYTVKD-CO2) and product peptide (for standard curve only) (NH2-KGGEEEEY-... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q2C53P5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Substrate (NH2-KGGEEEEYFELVKK-CO2), internal standard peptide (NH2-SWGAIETDKEYYTVKD-CO2) and product peptide (for standard curve only) (NH2-KGGEEEEY-... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q2C53P5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Substrate (NH2-KGGEEEEYFELVKK-CO2), internal standard peptide (NH2-SWGAIETDKEYYTVKD-CO2) and product peptide (for standard curve only) (NH2-KGGEEEEY-... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q2C53P5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK1 (aa 574-1154) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10487083 (2019) BindingDB Entry DOI: 10.7270/Q2SX6GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK2 (aa 532-1132) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10487083 (2019) BindingDB Entry DOI: 10.7270/Q2SX6GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK3 (aa 512-1124) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10487083 (2019) BindingDB Entry DOI: 10.7270/Q2SX6GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYK2 (aa 580-1182) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10487083 (2019) BindingDB Entry DOI: 10.7270/Q2SX6GM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK1 (aa 574-1154) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10981911 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK2 (aa 532-1132) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10981911 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| JAK3 (aa 512-1124) (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10981911 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYK2 [aa 580-1182,C936A,C1142A] (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description 11-point dosing series were made for each compound by serially diluting 1:3 or 1:4 in DMSO, with point 12 being a DMSO control. From the serial dilut... | US Patent US10981911 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 87.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM387827 (N-(4-(Cyanomethyl)bicyclo[2.2.1]heptan-1-yl)-2-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Substrate (NH2-KGGEEEEYFELVKK-CO2), internal standard peptide (NH2-SWGAIETDKEYYTVKD-CO2) and product peptide (for standard curve only) (NH2-KGGEEEEY-... | Bioorg Med Chem Lett 19: 773-7 (2009) BindingDB Entry DOI: 10.7270/Q2C53P5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||