

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CNCC(Cc1ccccc1F)c1ccc2ccccc2c1
InChI Key: InChIKey=DZNPDBNQTFEIMC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM388483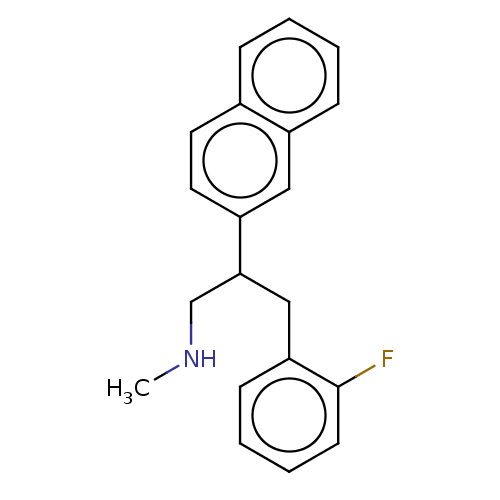 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description NET: This protocol was designed to measure inhibition of uptake by the human norepinephrine transporter. The reagents were human NET (HEK293F) cells,... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description SERT: This protocol was designed to measure inhibition of uptake by the human serotonin transporter. The reagents were human SERT (HEK293F) cells, fl... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description NET: This protocol was designed to measure inhibition of uptake by the human norepinephrine transporter. The reagents were human NET (HEK293F) cells,... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description SERT: This protocol was designed to measure inhibition of uptake by the human serotonin transporter. The reagents were human SERT (HEK293F) cells, fl... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description DAT: This protocol was designed to measure inhibition of uptake by the human dopamine transporter. The reagents were human DAT (HEK293F) cells, GBR 1... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description DAT: This protocol was designed to measure inhibition of uptake by the human dopamine transporter. The reagents were human DAT (HEK293F) cells, GBR 1... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description HERG: The pre- and post-compound hERG current was evoked by a single voltage pulse consisting of a 20 s period holding at −70 mV, a 160 ms step... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description HERG: The pre- and post-compound hERG current was evoked by a single voltage pulse consisting of a 20 s period holding at −70 mV, a 160 ms step... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM388483 (US9944618, Compound ID No. 17 | US9944618, Compoun...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||