

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM388521 US9944618, Compound ID No. 55
SMILES: CNC[C@@H]([C@H](O)c1cccnc1)c1ccc(OC)c(C)c1
InChI Key: InChIKey=VGYNYEFIVWGCPR-NVXWUHKLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM388521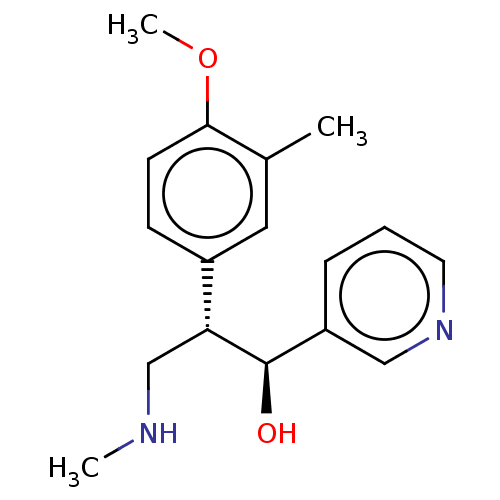 (US9944618, Compound ID No. 55) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description SERT: This protocol was designed to measure inhibition of uptake by the human serotonin transporter. The reagents were human SERT (HEK293F) cells, fl... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM388521 (US9944618, Compound ID No. 55) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description NET: This protocol was designed to measure inhibition of uptake by the human norepinephrine transporter. The reagents were human NET (HEK293F) cells,... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM388521 (US9944618, Compound ID No. 55) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description DAT: This protocol was designed to measure inhibition of uptake by the human dopamine transporter. The reagents were human DAT (HEK293F) cells, GBR 1... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM388521 (US9944618, Compound ID No. 55) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM388521 (US9944618, Compound ID No. 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Cytochrome P450 3A4 and 2D6:Recombinant enzymes, 3A4 and 2D6, generated using the ABL yeast expression system were used. For CYP3A4, the enzyme amou... | Bioorg Med Chem Lett 19: 1164-7 (2009) BindingDB Entry DOI: 10.7270/Q26D5WBR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||