

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM391726 N-[(2R)-3-(7-methyl-1H-::US10300056, Example 11::US10888561, Example 11
SMILES: Cc1cc(C[C@@H](NC(=O)N2CCC3(CC2)OC(=O)Nc2ncccc32)C(=O)N[C@@H](CC2CCNCC2)C(=O)N2CCN(CC2)c2ccncc2)cc2cn[nH]c12
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726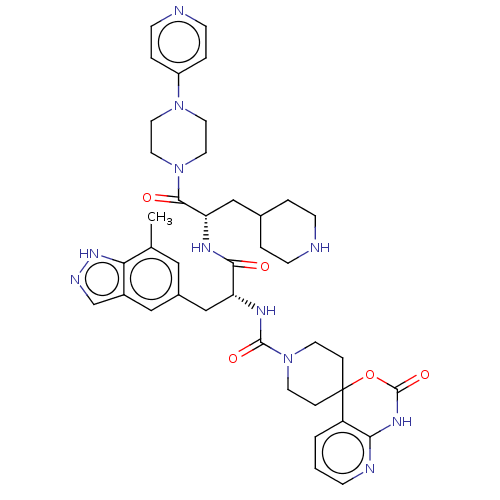 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin-gene-related peptide receptor, CALCRL/RAMP1 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited US Patent | Assay Description Radioligand binding assay. Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended i... | US Patent US10888561 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin-gene-related peptide receptor, CALCRL/RAMP1 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Human CGRP receptors expressed (consisting of CRLR and RAMP1) in insect Sf21 cell membrane homogenates were re-suspended in the binding buffer (10 mM... | Bioorg Med Chem Lett 18: 3641-5 (2008) BindingDB Entry DOI: 10.7270/Q2T155ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biohaven Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of human CLR/RAMP1 | J Med Chem 63: 6600-6623 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G protein-coupled receptor X2 (MRGPRX2) (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Agonist activity at MRGPX2 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM391726 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | <5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Inhibition of CYP2C19 (unknown origin) | J Med Chem 63: 7906-7920 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||