

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM398206 Preparations of 4-chloro-N-(((1R,3R)-1-hydroxy-3-methylcyclohexyl) methyl)-1-((tetrahydrofuran-2-yl)methyl)-1H-indole-3-carboxamide::US10323000, Compound 143::US10676433, Compound 143
SMILES: C[C@@H]1CCC[C@](O)(CNC(=O)c2cn(CC3CCCO3)c3cccc(Cl)c23)C1
InChI Key: InChIKey=BBQGQUYUBIGQSH-XIESDHNKSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM398206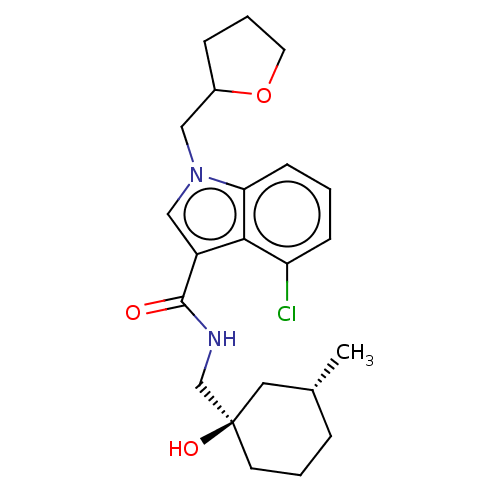 (Preparations of 4-chloro-N-(((1R,3R)-1-hydroxy-3-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg | Assay Description Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor.... | Bioorg Med Chem 16: 8574-86 (2008) BindingDB Entry DOI: 10.7270/Q26M395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 beta (Homo sapiens (Human)) | BDBM398206 (Preparations of 4-chloro-N-(((1R,3R)-1-hydroxy-3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to a... | US Patent US10676433 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM398206 (Preparations of 4-chloro-N-(((1R,3R)-1-hydroxy-3-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH US Patent | Assay Description Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor.... | US Patent US10676433 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 beta (Homo sapiens (Human)) | BDBM398206 (Preparations of 4-chloro-N-(((1R,3R)-1-hydroxy-3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-University Marburg | Assay Description The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to a... | Bioorg Med Chem 16: 8574-86 (2008) BindingDB Entry DOI: 10.7270/Q26M395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||