

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Nc1cc(Cc2cnn(Cc3ccc4CNCC(F)(F)c4c3)c2)c2nn[nH]c2n1
InChI Key: InChIKey=LMOPUOZPBAZXOU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloperoxidase (Homo sapiens (Human)) | BDBM413390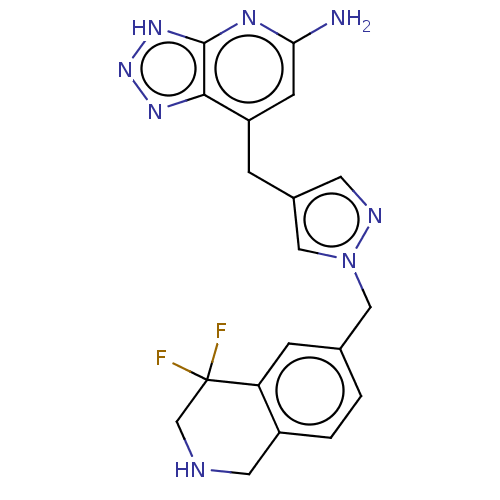 (US10407422, Example 260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10407422 (2019) BindingDB Entry DOI: 10.7270/Q28G8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||