

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCC[C@@H](C(=O)Nc1ccc(C(N)=O)c(F)c1)n1cc(OC)c(cc1=O)-c1cc(Cl)ccc1-n1cnnn1
InChI Key: InChIKey=CZWHJSKWMSOPRC-NRFANRHFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721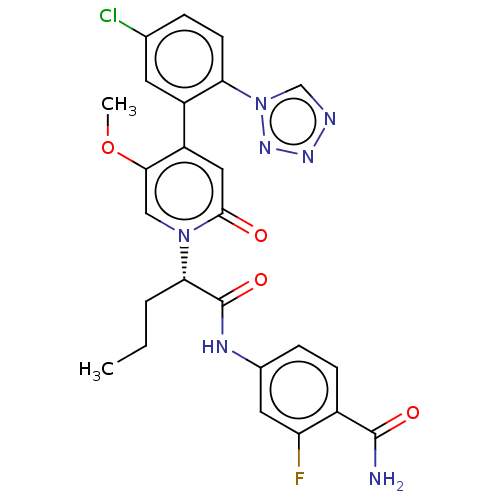 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413721 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413721 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||