

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1CCc2ccc(OCCCN3CCN(CC3)c3ccccc3Cl)cc2C1=O
InChI Key: InChIKey=QIMOBLHMKZLPIE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423312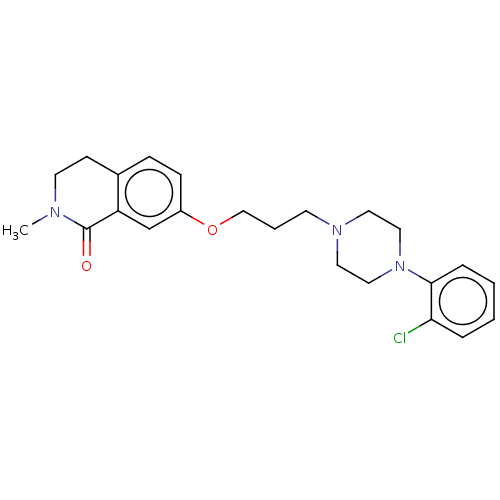 (US10501452, Compound 18) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H](+)8-OH-DPAT from rat cerebral cortex 5HT1A receptor measured after 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423312 (US10501452, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]ketanserin from rat cerebral cortex 5HT2A receptor measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM423312 (US10501452, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 391 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]spiperone from D2 receptor in rat striatum measured after 15 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112709 BindingDB Entry DOI: 10.7270/Q2XK8K6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM423312 (US10501452, Compound 18) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT1A: (1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed ... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM423312 (US10501452, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM423312 (US10501452, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description 5-HT2A:(1) The prepared membrane was applied with buffer, and homogenizer was used for evenly dispersing. 15 tubes were mixed into a 100 ml container... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||