

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cn1c(SCCCN2C[C@H]3CCN([C@H]3C2)c2ccc(cc2)C(F)(F)F)nnc1C1CCC(O)CC1
InChI Key: InChIKey=YHKSIIGCRXQDQA-CCMYNQISSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM435388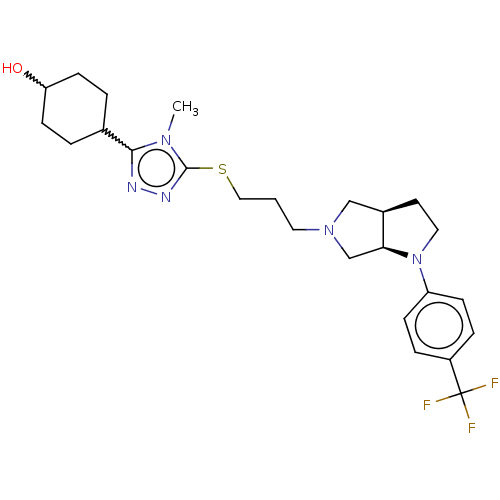 (US10584135, Example 219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description [3H]-Spiperone Binding Assay at hD3 and hD4 recombinant receptors CHO cells transiently transfected with human dopamine type 3 or 4 receptors (CHO-hD... | US Patent US10584135 (2020) BindingDB Entry DOI: 10.7270/Q29S1TFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM435388 (US10584135, Example 219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INDIVIOR UK LIMITED US Patent | Assay Description CHO cells stably expressing human dopamine receptor type 2, long variant (hD2L), coupled to Gα16 protein (CHO-Gα16-hD2L) were seeded into b... | US Patent US10584135 (2020) BindingDB Entry DOI: 10.7270/Q29S1TFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||