

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCNc1ncc2c(n1)N(C)CCN(c1cccc(O[C@@H](CCNC)c3ccccc3)c1)C2=O
InChI Key: InChIKey=MGGCXTKDCMPNFI-QHCPKHFHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM436373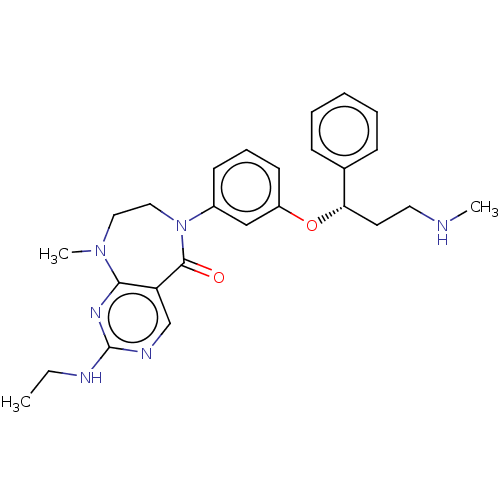 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-nisoxetine from human NET expressed in HEK293 cell membranes incubated for 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01867 BindingDB Entry DOI: 10.7270/Q2JW8JKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM436373 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE PHARMACEUTICALS, S.A. US Patent | Assay Description NET: Human norepinephrine transporter (NET) enriched membranes (5 μg) were incubated with 5 nM of radiolabeled [3H]-Nisoxetin in assay buffer co... | US Patent US10590140 (2020) BindingDB Entry DOI: 10.7270/Q2WM1HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent calcium channel subunit alpha-2/delta-1 [25-1103] (Homo sapiens (Human)) | BDBM436373 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE PHARMACEUTICALS, S.A. US Patent | Assay Description α2δ-1: Binding Assay to Human α2δ-1 Subunit of Cav2.2 Calcium Channel.Human α2δ-1 enriched membranes (2.5 μg) were... | US Patent US10590140 (2020) BindingDB Entry DOI: 10.7270/Q2WM1HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent calcium channel subunit alpha-2/delta-1 [25-1103] (Homo sapiens (Human)) | BDBM436373 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ESTEVE PHARMACEUTICALS, S.A. US Patent | Assay Description α2δ-1: Binding Assay to Human α2δ-1 Subunit of Cav2.2 Calcium Channel.Human α2δ-1 enriched membranes (2.5 μg) were... | US Patent US10590140 (2020) BindingDB Entry DOI: 10.7270/Q2WM1HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent calcium channel subunit alpha-2/delta-1 (Homo sapiens (Human)) | BDBM436373 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-gabapentin from human Cav alpha2delta1 expressed in CHO-K1 cell membranes incubated for 60 mins by scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01867 BindingDB Entry DOI: 10.7270/Q2JW8JKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM436373 ((S)-2-(ethylamino)-9- methyl-6-(3-(3- (methylamino...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NET expressed in HEK293 cells assessed as reduction in ASP+ uptake incubated for 20 mins before ASP+ addition and measured after ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01867 BindingDB Entry DOI: 10.7270/Q2JW8JKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||