

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM441777 US10640533, Identification number CX13-109::US10640533, Table 1.24
SMILES: CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccncc1)C(=O)[C@@]1(C)CO1
InChI Key: InChIKey=SDKAVNLYMZVQEF-ZPBGSLHZSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441777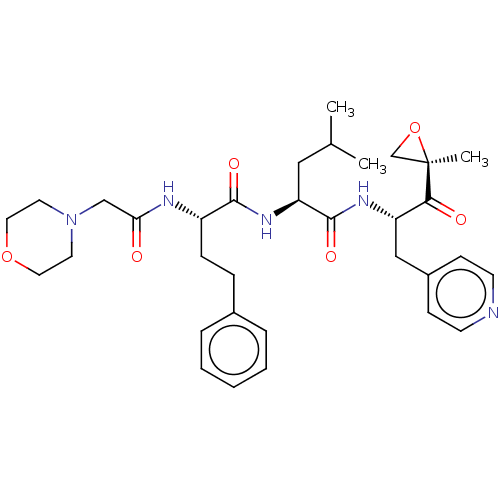 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 11D (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane protease serine 11D (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||