

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@@H]1O[C@H](C[C@H](O)[C@@H]1O[C@H]1C[C@H](O)[C@H](OC2CCN(CC(C)O2)C2CC2)[C@H](C)O1)O[C@H]1CC[C@@]2(C)C(CCC3C2C[C@H](O)[C@]2(C)[C@H](CC[C@]32O)C2=CC(=O)OC2)C1
InChI Key: InChIKey=LJUOSQZLMDHEKF-DEGSDRIYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium/potassium-transporting ATPase subunit alpha-2/beta-3 (Homo sapiens (Human)) | BDBM444980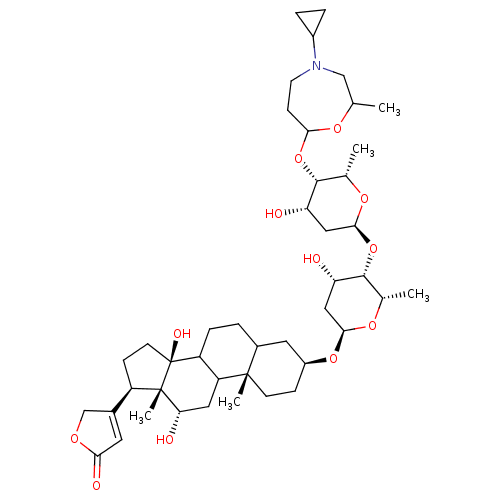 (US10668094, Compound DcP-(cyclic)) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-2/beta-2 (Homo sapiens (Human)) | BDBM444980 (US10668094, Compound DcP-(cyclic)) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-2/beta-1 (Homo sapiens (Human)) | BDBM444980 (US10668094, Compound DcP-(cyclic)) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM444980 (US10668094, Compound DcP-(cyclic)) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yeda Research and Development Co. Ltd. US Patent | Assay Description To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isofo... | US Patent US10668094 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||