

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1ccncc1-c1ccc(cc1)N1CCCC(NC(=O)Nc2ccc(cc2)C(F)(F)F)C1=O
InChI Key: InChIKey=QRBKHAVJURUOIT-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-formyl peptide receptor 2 (Homo sapiens (Human)) | BDBM454183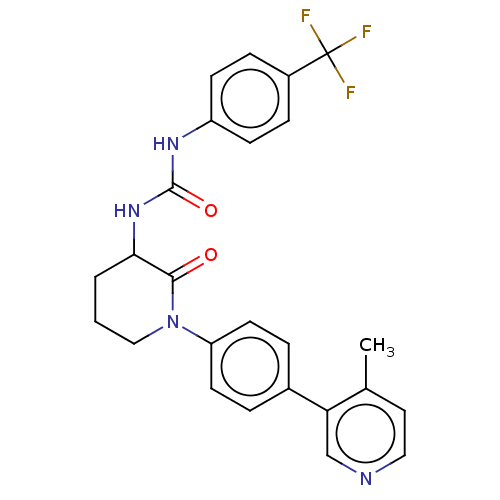 (US10717708, Example 47 | US11186544, Example 47) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FPR2 and FPR1 Cyclic Adenosine Monophosphate (cAMP) Assays. A mixture of forskolin (5 μM final for FPR2 or 10 μM final for FPR1) and IBMX (... | US Patent US10717708 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM454183 (US10717708, Example 47 | US11186544, Example 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-formyl peptide receptor 2 (Homo sapiens (Human)) | BDBM454183 (US10717708, Example 47 | US11186544, Example 47) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM454183 (US10717708, Example 47 | US11186544, Example 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FPR2 and FPR1 Cyclic Adenosine Monophosphate (cAMP) Assays. A mixture of forskolin (5 μM final for FPR2 or 10 μM final for FPR1) and IBMX (... | US Patent US10717708 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||