

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3C(O)CCCc23)c(Cl)cn1
InChI Key: InChIKey=BRQSWVAJJNFGEP-IODPJOFQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM454593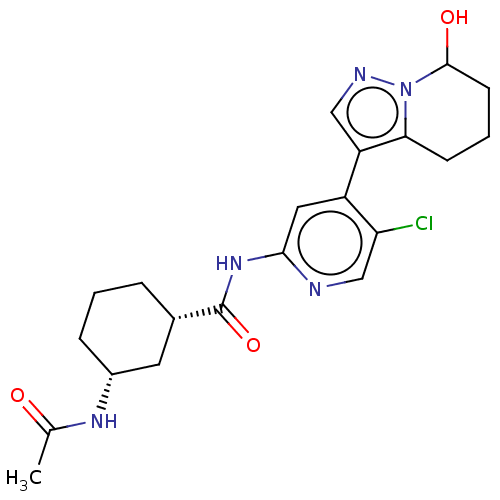 (US10717746, Example 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Activity of CDK9 was determined in-vitro using a mobility shift assay on a Caliper LC3000 reader (Caliper/PerkinElmer), which measures fluorescence o... | US Patent US10717746 (2020) BindingDB Entry DOI: 10.7270/Q2NV9N9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM454593 (US10717746, Example 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK9 in human MCF7 cells assessed as reduction in RNA Polymerase 2 CTD phosphorylation at Ser2 residues measured after 6 hrs by immunof... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01754 BindingDB Entry DOI: 10.7270/Q2M90D8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM454593 (US10717746, Example 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant full length human N-terminal GST-fused CDK9 (1 to 372 residues)/His-tagged CyclinT1 (1 to 726 residues) expressed in baculo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01754 BindingDB Entry DOI: 10.7270/Q2M90D8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM454593 (US10717746, Example 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Activity of CDK9 was determined in-vitro using a mobility shift assay on a Caliper LC3000 reader (Caliper/PerkinElmer), which measures fluorescence o... | US Patent US10717746 (2020) BindingDB Entry DOI: 10.7270/Q2NV9N9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||