

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCC(CC)n1cc(cn1)-c1nc(cn2nccc12)-c1cnn(c1)C1CC(C1)S(C)(=O)=O
InChI Key: InChIKey=TXUGWEWYIDJKMN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM455371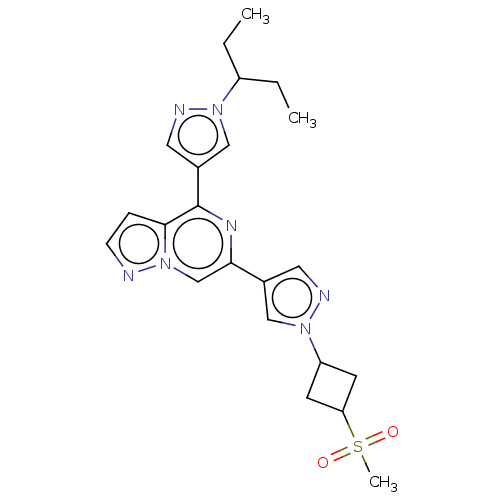 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Tyk2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM455371 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK3 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM455371 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM455371 (6-(1-(1-(methylsulfonyl)azetidin-3-yl)-1H-pyrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.; Celgene Corporation US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit JAK1 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US10730880 (2020) BindingDB Entry DOI: 10.7270/Q2CC13R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||