

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)(O)Cn1nnc2cc(c(F)c(C#N)c12)-c1ccc(cc1-c1ccc(C#N)c(F)c1)C(=O)N1[C@@H]2CC[C@H]1[C@@H](N)C2
InChI Key: InChIKey=XZFTUDMYCBPHQN-KAZGAHJFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM456518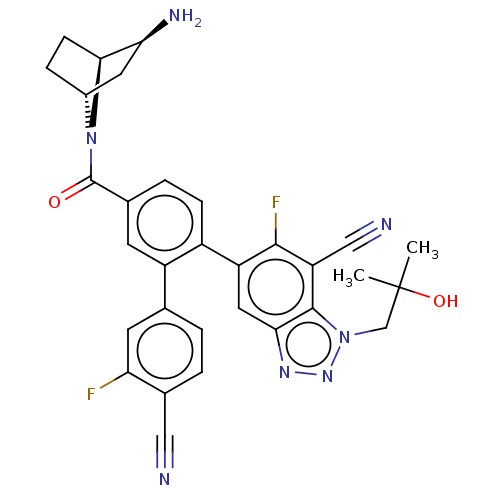 (US10723742, Example 247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The conditions for measuring inhibitory activity of compounds against LSD1 activity were determined with reference to a document available from the w... | US Patent US10723742 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||