

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Fc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM471715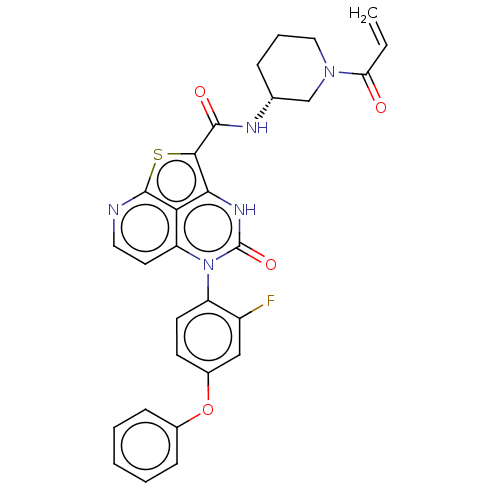 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Covalent inhibition of recombinant human GST-tagged BTK (2 to 659 end residues) expressed in baculovirus expression system assessed as inhibition con... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Mus musculus) | BDBM471715 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BTK in C57Bl/6 mouse splenocyte assessed as reduction in anti-IgM-induced CD69 expression incubated for 1 hr followed anti-IgM stimulat... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00044 BindingDB Entry DOI: 10.7270/Q25D8WM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK [386-659] (Homo sapiens (Human)) | BDBM471715 ((R)-N-(1-Acryloylpiperidin-3-yl)-5-(2-fluoro-4-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description A BTK kinase lanthascreen binding assay monitors compound binding to unphosphorylated-BTK kinase domain (UP-BTK), by competing with a fluorescent lab... | US Patent US10822348 (2020) BindingDB Entry DOI: 10.7270/Q22V2K6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||