

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COC(C)Cc1ccc(NS(=O)(=O)C2=CC3C(C=C2Br)C(C)(C)CCC3(C)C)cc1
InChI Key: InChIKey=KTKWRYFUVKVPFK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 26A1 (Homo sapiens (Human)) | BDBM476221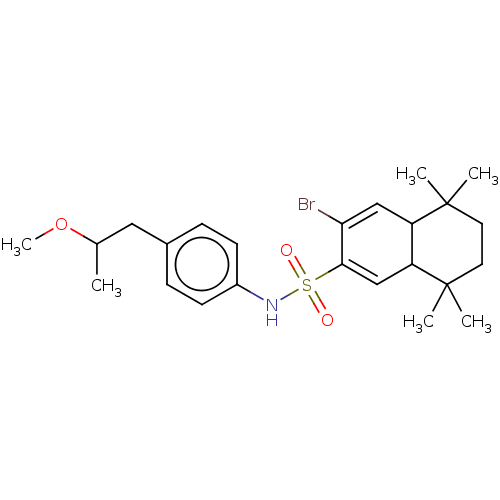 (US10874634, Cmpd No. 04B) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen''s University at Kingston US Patent | Assay Description CYP26A1 or CYP26B1 stably transfected HeLa cells were maintained in Minimum Essential Medium (MEM) containing 10% fetal bovine serum (FBS) and 100 &#... | US Patent US10874634 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||