

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: F[C@@H]1CCN(Cc2cc(Cl)c(O[C@H]3CCc4c3cccc4-c3cccc(OCCCN4CCC(F)CC4)c3Cl)cc2OCc2cncc(c2)C#N)C1
InChI Key: InChIKey=FGWOTXVFFFYRCH-XLNFBEDTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Programmed cell death 1 ligand 1 (Homo sapiens (Human)) | BDBM482462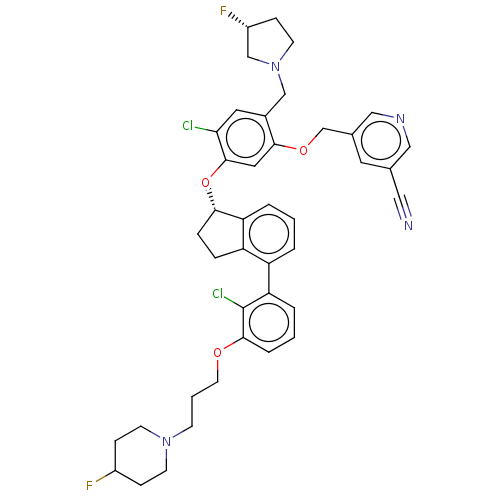 (US10919852, Compound TABLE 1.161 | US11708326, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q22N56DJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Programmed cell death protein 1 (Homo sapiens) | BDBM482462 (US10919852, Compound TABLE 1.161 | US11708326, Com...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemoCentryx, Inc. US Patent | Assay Description 96 Well plates were coated with 1 g/mL of human PD-L1 (obtained from R&D) in PBS overnight at 4° C. The wells were then blocked with 2% BSA in PBS (W... | US Patent US10919852 (2021) BindingDB Entry DOI: 10.7270/Q2RF5Z3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||