

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NS(=O)(=O)c1ccc(Cc2cn(nc2-c2cccc(OCc3ccccn3)c2)-c2nc(cs2)C(O)=O)cc1
InChI Key: InChIKey=FDUIVDAUBFHRSL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489086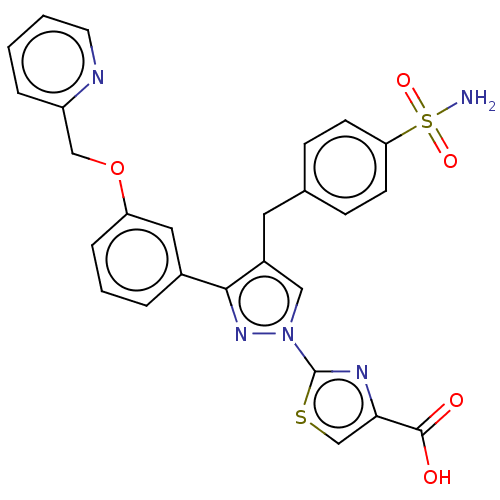 (2-(3-(3-(pyridin-3- ylmethoxy)phenyl)-4- (4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES; VANDERBILT UNIVERSITY; THE UAB RESEARCH FOUNDATION; THE TRUSTEES OF THE UNIVERSITY OF PENNSYLVANIA US Patent | Assay Description Test compounds were placed in a Greiner Bio-One (Monroe, N.C.) 1536-well black solid bottom assay plate. 200 millimolar (mM) Tris HCl, pH 7.4, 100 mi... | US Patent US10961200 (2021) BindingDB Entry DOI: 10.7270/Q2474DZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489086 (2-(3-(3-(pyridin-3- ylmethoxy)phenyl)-4- (4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LDHA in human MIA PaCa2 cells assessed as reduction in lactate production incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM489086 (2-(3-(3-(pyridin-3- ylmethoxy)phenyl)-4- (4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human LDHA using sodium pyruvate as substrate preincubated for 5 mins followed by diaphorase/resazurin addition and measure... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127974 BindingDB Entry DOI: 10.7270/Q24J0JV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||