

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50005826 CHEMBL286843::[2-(1H-Indol-3-yl)-1-methyl-1-(2-phenethyl-4,5-dihydro-thiazol-5-yl)-ethyl]-carbamic acid adamantan-2-yl ester
SMILES: CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C1SC(CCc2ccccc2)C=N1
InChI Key: InChIKey=COBAVOQKOJMCCB-YCHNYHORSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor (MOUSE) | BDBM50005826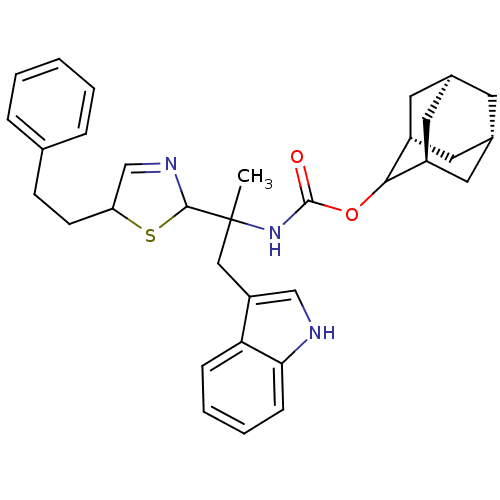 (CHEMBL286843 | [2-(1H-Indol-3-yl)-1-methyl-1-(2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 827 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Center Curated by ChEMBL | Assay Description Inhibition of [125I]CCK-8 binding to cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 35: 1472-84 (1992) BindingDB Entry DOI: 10.7270/Q2H41S3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50005826 (CHEMBL286843 | [2-(1H-Indol-3-yl)-1-methyl-1-(2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 801 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Center Curated by ChEMBL | Assay Description Inhibition of [125I]CCK-8 binding to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 35: 1472-84 (1992) BindingDB Entry DOI: 10.7270/Q2H41S3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||