

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50010465 CHEMBL3263996
SMILES: CNS(=O)(=O)Nc1cc(Cc2c(C)c3ccc(OC(=O)N(C)C)cc3oc2=O)ccn1
InChI Key: InChIKey=GLVLHQQZXXQWEI-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50010465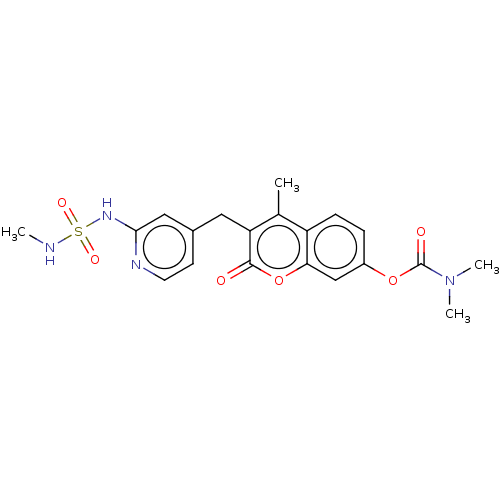 (CHEMBL3263996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of ... | ACS Med Chem Lett 5: 309-14 (2014) Article DOI: 10.1021/ml400379x BindingDB Entry DOI: 10.7270/Q27W6DQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50010465 (CHEMBL3263996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of... | ACS Med Chem Lett 5: 309-14 (2014) Article DOI: 10.1021/ml400379x BindingDB Entry DOI: 10.7270/Q27W6DQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50010465 (CHEMBL3263996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of truncated active C-Raf (unknown origin) assessed as MEK1 phosphorylation using inactive MEK1 K97R as substrate after 45 mins | ACS Med Chem Lett 5: 309-14 (2014) Article DOI: 10.1021/ml400379x BindingDB Entry DOI: 10.7270/Q27W6DQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50010465 (CHEMBL3263996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in presence of ... | ACS Med Chem Lett 5: 309-14 (2014) Article DOI: 10.1021/ml400379x BindingDB Entry DOI: 10.7270/Q27W6DQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50010465 (CHEMBL3263996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 30 mins preincubation by LC-MS/MS analysis in absence of N... | ACS Med Chem Lett 5: 309-14 (2014) Article DOI: 10.1021/ml400379x BindingDB Entry DOI: 10.7270/Q27W6DQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||