

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50014075 (3R,4S)-3,4-Dihydroxy-pyrrolidine-1-carboxylic acid [(S)-1-[(S)-1-[(1S,2R)-1-cyclohexylmethyl-2-((S)-3-ethyl-2-oxo-oxazolidin-5-yl)-2-hydroxy-ethylcarbamoyl]-2-(3H-imidazol-4-yl)-ethylcarbamoyl]-2-(4-methoxy-phenyl)-ethyl]-amide::CHEMBL61428
SMILES: CCN1C[C@H](OC1=O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)N1C[C@H](O)[C@H](O)C1
InChI Key: InChIKey=QDIKJNAKIBUDPQ-XJHZBCOISA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50014075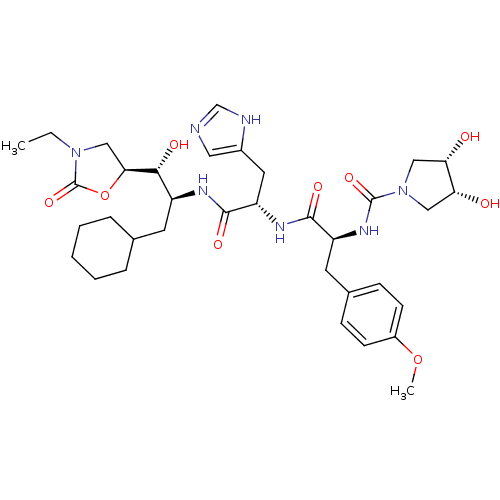 ((3R,4S)-3,4-Dihydroxy-pyrrolidine-1-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin at pH 7.4 | J Med Chem 33: 1962-9 (1990) BindingDB Entry DOI: 10.7270/Q2KW5GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50014075 ((3R,4S)-3,4-Dihydroxy-pyrrolidine-1-carboxylic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against purified human renal renin at pH 6.5 | J Med Chem 33: 1962-9 (1990) BindingDB Entry DOI: 10.7270/Q2KW5GN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||