

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50019202 7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihydro-benzo[e][1,4]diazepin-2-one::CCK antagonist synthetic 5::CHEMBL70728
SMILES: Clc1ccc2NC(=O)[C@@H](Cc3c[nH]c4ccccc34)N=C(c3ccccc3)c2c1
InChI Key: InChIKey=OAAIQFQHSUFGKD-JOCHJYFZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin (RAT) | BDBM50019202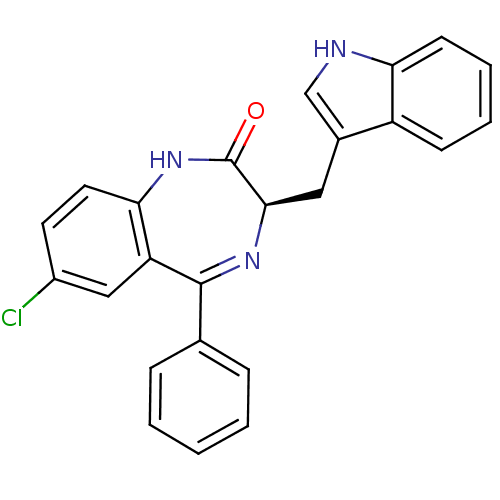 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 4918-22 (1986) Article DOI: 10.1073/pnas.83.13.4918 BindingDB Entry DOI: 10.7270/Q2445K05 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Rattus norvegicus (Rat)) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 4918-22 (1986) Article DOI: 10.1073/pnas.83.13.4918 BindingDB Entry DOI: 10.7270/Q2445K05 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Mus musculus) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin receptor | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]gastrin binding to guinea pig gastric glands | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]-CCK-33 binding to rat pancreas cholecystokinin receptor | J Med Chem 30: 1229-39 (1987) BindingDB Entry DOI: 10.7270/Q2SF2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin receptor from guinea pig brain tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (Homo sapiens (Human)) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Half-maximal inhibition of binding of [125I]gastrin to guinea pig gastric glands | J Med Chem 30: 1229-39 (1987) BindingDB Entry DOI: 10.7270/Q2SF2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin A receptor (Cavia porcellus) | BDBM50019202 (7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-33 binding to guinea pig brain(cortex) cholecystokinin receptor | J Med Chem 30: 1229-39 (1987) BindingDB Entry DOI: 10.7270/Q2SF2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||