

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50029577 2-{2-[1-(4-Chloro-benzyl)-4-methyl-6-(5-phenyl-pyridin-2-ylmethoxy)-4,5-dihydro-1H-thiopyrano[2,3,4-cd]indol-2-yl]-ethoxy}-butyric acid::CHEMBL275634
SMILES: CCC(OCCc1c2SC(C)Cc3c(OCc4ccc(cn4)-c4ccccc4)ccc(n1Cc1ccc(Cl)cc1)c23)C(O)=O
InChI Key: InChIKey=JCBSPQXPBDDSEZ-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029577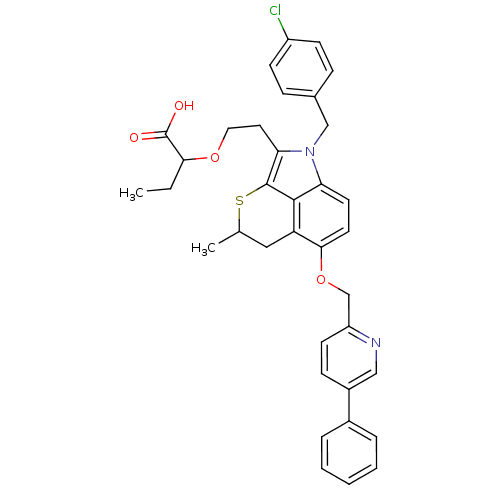 (2-{2-[1-(4-Chloro-benzyl)-4-methyl-6-(5-phenyl-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of human 5-Lipoxygenase. | J Med Chem 37: 1153-64 (1994) BindingDB Entry DOI: 10.7270/Q2PV6JF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029577 (2-{2-[1-(4-Chloro-benzyl)-4-methyl-6-(5-phenyl-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro inhibition of human 5-Lipoxygenase. | J Med Chem 37: 1153-64 (1994) BindingDB Entry DOI: 10.7270/Q2PV6JF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50029577 (2-{2-[1-(4-Chloro-benzyl)-4-methyl-6-(5-phenyl-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Human 5-lipoxygenase | J Med Chem 38: 4538-47 (1995) BindingDB Entry DOI: 10.7270/Q2C53JW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||