

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50030683 CHEMBL121907::N-Butyl-3-[4-(3-fluoro-2'-(N-t-butyloxycarbonyl)-Sulfanamido-4-ylmethyl)-5-oxo-3-propyl-4,5-dihydro-[1,2,4]triazol-1-yl]-4-trifluoromethyl-benzamide
SMILES: CCCCNC(=O)c1ccc(c(c1)-n1nc(CCC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O)C(F)(F)F
InChI Key: InChIKey=GQEYIVWYNBRECD-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50030683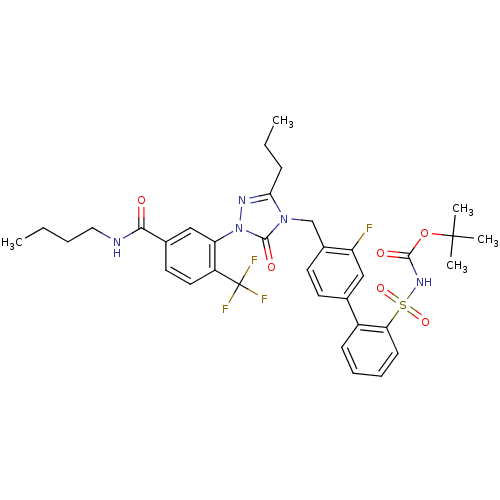 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II AT2 (RAT) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor, type 2 in rat midbrain membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II receptor type 1, in human adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.441 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Oryctolagus cuniculus (rabbit) aortic AT1 receptor | Citation and Details Article DOI: 10.1007/s00044-011-9815-x BindingDB Entry DOI: 10.7270/Q2VT1W0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II AT2 (RAT) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II type 1a (AT-1a) receptor (RABBIT) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at Oryctolagus cuniculus (rabbit) aortic AT1 receptor | Citation and Details Article DOI: 10.1007/s00044-011-9815-x BindingDB Entry DOI: 10.7270/Q2VT1W0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin II receptor (Homo sapiens (Human)) | BDBM50030683 (CHEMBL121907 | N-Butyl-3-[4-(3-fluoro-2'-(N-t-buty...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin II receptor type 2 in human adrenal membrane preparations. | J Med Chem 38: 3741-58 (1995) BindingDB Entry DOI: 10.7270/Q2Q81C35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||