

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50044079 5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)-ylidene]-4-oxo-4,5-dihydro-thiazol-2-yl-cyanamide::CHEMBL16048
SMILES: CC(C)(C)c1cc(C=C2SC(NC#N)=NC2=O)cc(c1O)C(C)(C)C
InChI Key: InChIKey=QHJVYZBKWNABSG-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50044079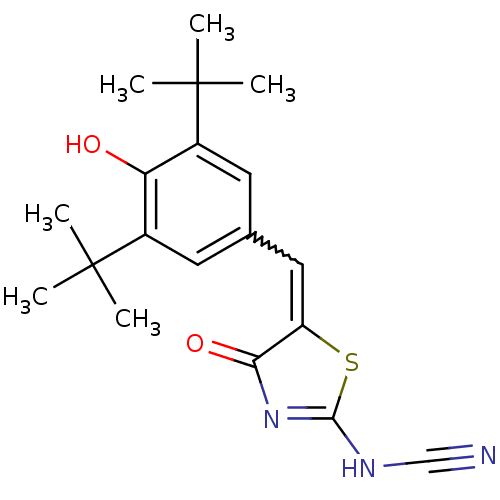 (5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in intact rat basophilic leukemia cells stimulated with the calciumionophore A-23,187 | J Med Chem 37: 322-8 (1994) BindingDB Entry DOI: 10.7270/Q28C9V9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50044079 (5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against recombinant human Prostaglandin G/H synthase 2 | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-2 (COX-2) (Mus musculus (Mouse)) | BDBM50044079 (5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 value was determined against Prostaglandin G/H synthase 2 of J7744A.1 cell lines. | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50044079 (5-[1-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-meth-(Z)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description IC50 against Prostaglandin G/H synthase 1 from human platelet rich plasma | J Med Chem 42: 1151-60 (1999) Article DOI: 10.1021/jm9805081 BindingDB Entry DOI: 10.7270/Q24X56Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||