

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049059 CHEMBL3319503::US10730833, Compound 10a::US9969686, Compound 10a
SMILES: Clc1ccc2[nH]cc(Cc3cc4ccccc4[nH]3)c2c1
InChI Key: InChIKey=OSTIXKKSSZMBPY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059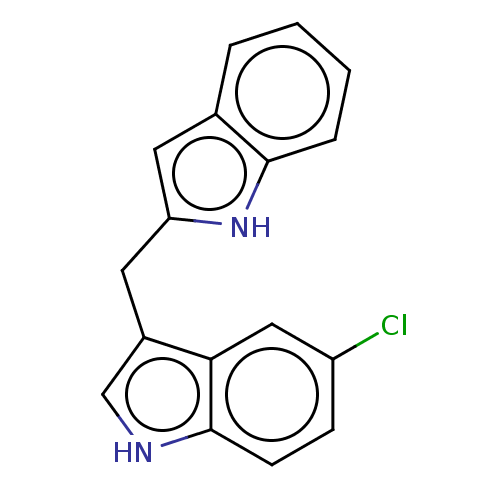 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Activation of AhR in human HepG2 cells assessed as fluorescence after 24 hrs incubation by EROD assay | Bioorg Med Chem Lett 24: 4023-5 (2014) Article DOI: 10.1016/j.bmcl.2014.06.009 BindingDB Entry DOI: 10.7270/Q2TX3H0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibition of human PCSK9 in HepG2 cells incubated for 48 hrs by AlphaLISA assay | Bioorg Med Chem Lett 29: 2345-2348 (2019) Article DOI: 10.1016/j.bmcl.2019.06.014 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
Wisconsin Alumni Research Foundation US Patent | Assay Description This assay measures the induction of cytochrome P450-1A1 (CYP1A1), which is a major outcome of AhR activation. Whyte, J. J.; Jung, R. E.; Schmitt, C.... | US Patent US10730833 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50049059 (CHEMBL3319503 | US10730833, Compound 10a | US99696...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | >6.70E+3 | n/a | n/a | n/a | n/a |
University of St Andrews | Assay Description AhR readily binds to various endogenous and xenobiotic polyaromatic heterocycles. | J Med Chem 52: 2673-82 (2009) BindingDB Entry DOI: 10.7270/Q2DN47DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||