

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049215 1-Phosphono-4-[3-(2-benzylphenoxy)phenyl]butylsulfonic Acid Tripotassium Salt::CHEMBL160318::Tripotassium salt of4-[3-(2-Benzyl-phenoxy)-phenyl]-1-phosphono-butane-1-sulfonic acid
SMILES: [O-]P([O-])(=O)C(CCCc1cccc(Oc2ccccc2Cc2ccccc2)c1)S([O-])(=O)=O
InChI Key: InChIKey=ZMOCESLEEQXYRI-UHFFFAOYSA-K
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthetase (Rattus norvegicus) | BDBM50049215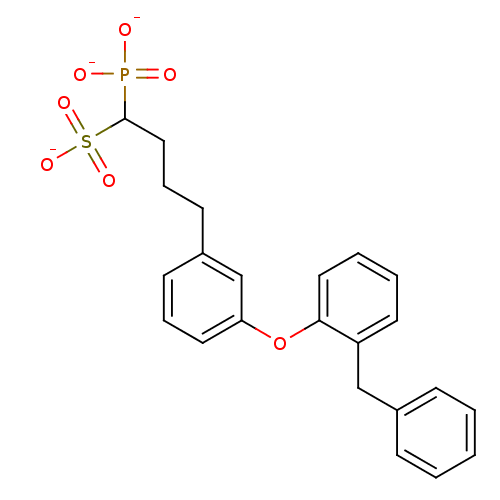 (1-Phosphono-4-[3-(2-benzylphenoxy)phenyl]butylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthetase (Homo sapiens (Human)) | BDBM50049215 (1-Phosphono-4-[3-(2-benzylphenoxy)phenyl]butylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human recombinant squalene synthase expressed in Escherichia coli BL21 (DE3) cells assessed as formation of 1,10-dioic acid metabolite ... | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dehydrosqualene synthase (Staphylococcus aureus) | BDBM50049215 (1-Phosphono-4-[3-(2-benzylphenoxy)phenyl]butylsulf...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay | J Med Chem 52: 976-88 (2009) Article DOI: 10.1021/jm801023u BindingDB Entry DOI: 10.7270/Q2VM4C87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||