

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50051864 (1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-6-phenyl-hexyl)-6-((E)-(4S,6S)-4,6-dimethyl-oct-2-enoyloxy)-4,7-dihydroxy-3-hydroxymethyl-2,8-dioxa-bicyclo[3.2.1]octane-4,5-dicarboxylic acid::CHEMBL30572
SMILES: CC[C@H](C)C[C@H](C)\C=C\C(=O)O[C@@H]1[C@@H](O)[C@]2(CCC(=C)[C@@H](OC(C)=O)[C@H](C)Cc3ccccc3)O[C@@]1(C(O)=O)[C@@](O)([C@@H](CO)O2)C(O)=O
InChI Key: InChIKey=HVJXODUTFFLCJZ-KLNZUXGYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthetase (Rattus norvegicus) | BDBM50051864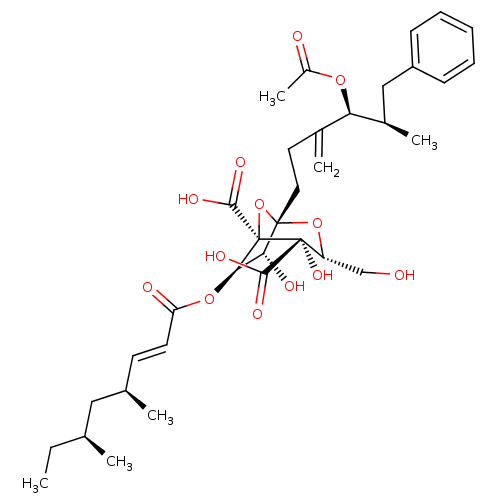 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of rat squalene synthase | Bioorg Med Chem Lett 4: 1931-1936 (1994) Article DOI: 10.1016/S0960-894X(01)80537-0 BindingDB Entry DOI: 10.7270/Q2D50MW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthetase (Rattus norvegicus) | BDBM50051864 ((1S,3R,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against Squalene synthase from rats | J Med Chem 39: 1413-22 (1996) Article DOI: 10.1021/jm950893j BindingDB Entry DOI: 10.7270/Q2Z0377G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||