

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50052127 Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-6-propylcarbamoyloxy-3-vinyl-dodecahydro-benzo[f]chromen-5-yl ester::CHEMBL329916
SMILES: CCCNC(=O)O[C@@H]1[C@H](OC(C)=O)[C@@]2(C)O[C@](C)(CC(=O)[C@]2(O)[C@@]2(C)[C@@H](O)CCC(C)(C)[C@H]12)C=C
InChI Key: InChIKey=LUAMGWQEEINJGO-VYHDIKPLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenylate cyclase (Homo sapiens (Human)) | BDBM50052127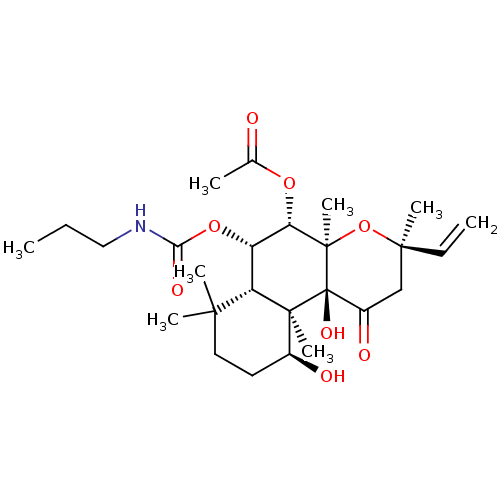 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Ability to activate the conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase (Homo sapiens (Human)) | BDBM50052127 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Conversion of [32P] ATP to [32P]-cyclic AMP mediated by adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase (Homo sapiens (Human)) | BDBM50052127 (Acetic acid (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-10,10...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Food and Drug Administration Curated by ChEMBL | Assay Description Inhibition of [125 I]-6-IHPP-forskolin binding to adenylate cyclase 1 | J Med Chem 39: 2745-52 (1996) Article DOI: 10.1021/jm960191+ BindingDB Entry DOI: 10.7270/Q2X34WJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||