

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50056678 CHEMBL3341792
SMILES: Nc1ncc(cc1-c1ccnc2ccccc12)-c1cccc(c1)N1CCNCC1
InChI Key: InChIKey=OWGBNDVAAXWRCD-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50056678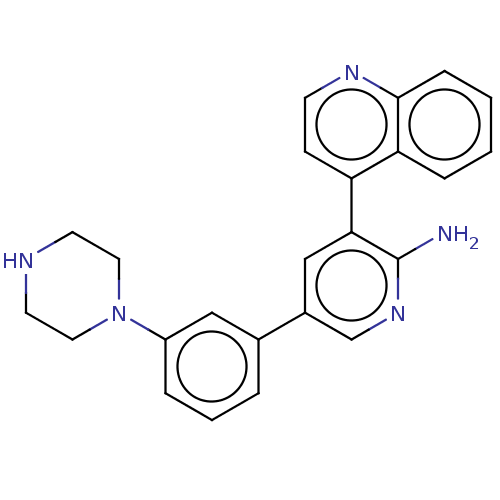 (CHEMBL3341792) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant human ALK2 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P] | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50056678 (CHEMBL3341792) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of TGFbeta1-induced TGFbeta type 1 ALK5 in HEK293T cells after 30 mins by luciferase reporter gene assay | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK2 (Mus musculus) | BDBM50056678 (CHEMBL3341792) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of BMP6-induced BMP receptor type 1 ALK2 in mouse C2C12 cells after 30 mins by luciferase reporter gene assay | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50056678 (CHEMBL3341792) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts Institute of Technology Curated by ChEMBL | Assay Description Inhibition of purified human ALK5 kinase after 45 mins by liquid scintillation counting in presence of ATP [gamma-32P] | J Med Chem 57: 7900-15 (2014) Article DOI: 10.1021/jm501177w BindingDB Entry DOI: 10.7270/Q2PK0HS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||