

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50070104 (2E)-3-(3-Hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one::(E)-3-(3''-Hydroxy-4''-methoxyphenyl)-1-(3',4',5'-trimethoxyphenyl)prop-2-en-1-one::(E)-3-(3''-hydroxy-4''-methoxyphenyl)-1-(3',4',5'-trimethoxyphenyl)-2-propen-1-one::(E)-3-(3-Hydroxy-4-methoxy-phenyl)-1-(3,4,5-trimethoxy-phenyl)-propenone::(E)-3-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one::3-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one::3-hydroxy-3',4,4',5'-tetramethoxychalcone::CHEMBL9941
SMILES: COc1ccc(\C=C\C(=O)c2cc(OC)c(OC)c(OC)c2)cc1O
InChI Key: InChIKey=IKMOZUDCUBMIRL-FNORWQNLSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50070104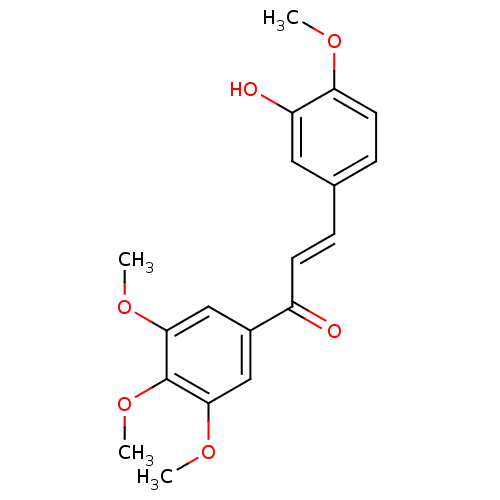 ((2E)-3-(3-Hydroxy-4-methoxyphenyl)-1-(3,4,5-trimet...) | MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calabria Curated by ChEMBL | Assay Description Inhibition of ACE in rabbit lung assessed as decrease in dansylglycine concentration after 5 mins by HPLC analysis | Bioorg Med Chem Lett 20: 1990-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.113 BindingDB Entry DOI: 10.7270/Q2G73DW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta-1 chain (Homo sapiens (Human)) | BDBM50070104 ((2E)-3-(3-Hydroxy-4-methoxyphenyl)-1-(3,4,5-trimet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salford Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of tubulin polymerization | J Med Chem 48: 457-65 (2005) Article DOI: 10.1021/jm049444m BindingDB Entry DOI: 10.7270/Q2416WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||