

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50072191 1,2,5,6-Tetrahydro-pyrido[1,2-a]quinolin-3-one::1,2,5,6-tetrahydro pyrido[1,2-a]quinolin-3-one::CHEMBL96006
SMILES: O=C1CCN2C(CCc3ccccc23)=C1
InChI Key: InChIKey=OPJKPSOLCGGXEW-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50072191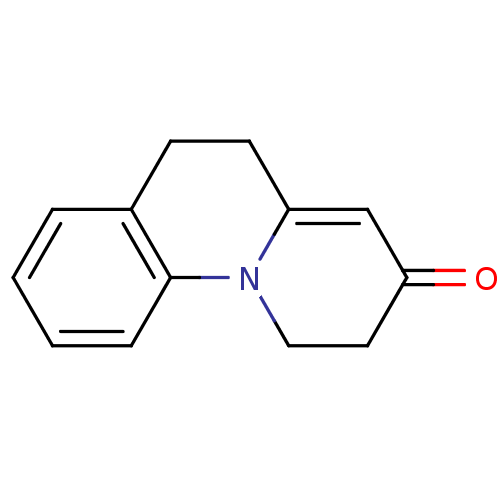 (1,2,5,6-Tetrahydro-pyrido[1,2-a]quinolin-3-one | 1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Firenze Curated by ChEMBL | Assay Description Inhibition of human Steroid 5-alpha-reductase type I | Bioorg Med Chem Lett 10: 353-6 (2000) BindingDB Entry DOI: 10.7270/Q2K35SVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50072191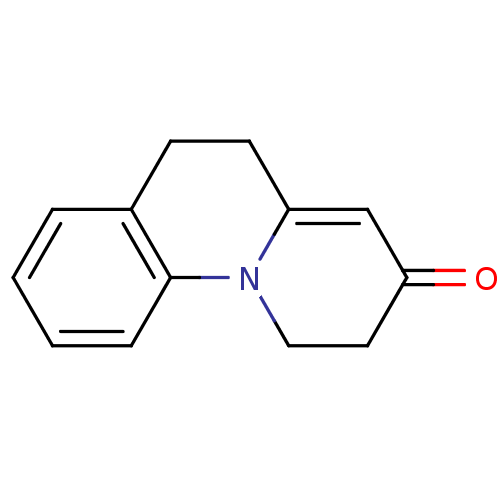 (1,2,5,6-Tetrahydro-pyrido[1,2-a]quinolin-3-one | 1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I expressed in CHO cells | J Med Chem 43: 3718-35 (2000) BindingDB Entry DOI: 10.7270/Q25B036J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50072191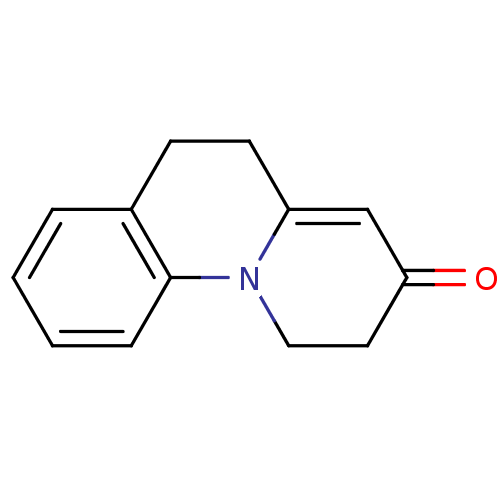 (1,2,5,6-Tetrahydro-pyrido[1,2-a]quinolin-3-one | 1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of human Steroid 5-alpha-reductase type 1 from recombinant CHO cells | Bioorg Med Chem Lett 8: 2871-6 (1999) BindingDB Entry DOI: 10.7270/Q2CC1169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5α-Reductase 1 (5α-R1) (Homo sapiens (Human)) | BDBM50072191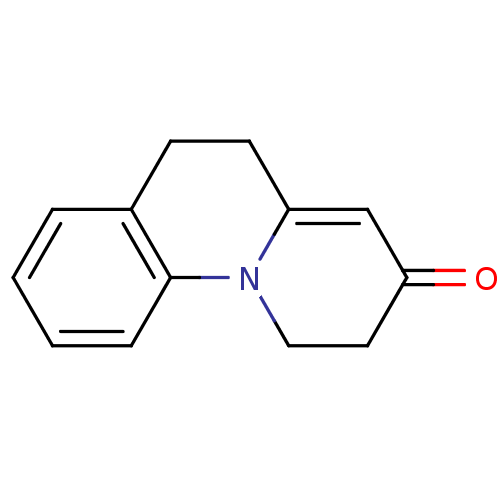 (1,2,5,6-Tetrahydro-pyrido[1,2-a]quinolin-3-one | 1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant steroid 5-alpha-reductase type I in stably transfected chinese hamster ovary (CHO) 1827 cells usin... | J Med Chem 47: 3546-60 (2004) Article DOI: 10.1021/jm031131o BindingDB Entry DOI: 10.7270/Q24J0DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||