

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50072715 9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-aza-chrysene::9-fluoro-2,2,4-trimethyl-2,5-dihydro-1H-chromeno[3,4-f]quinoline::CHEMBL65079
SMILES: CC1=CC(C)(C)Nc2ccc-3c(COc4ccc(F)cc-34)c12
InChI Key: InChIKey=GXFYVOIWLKLUCK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715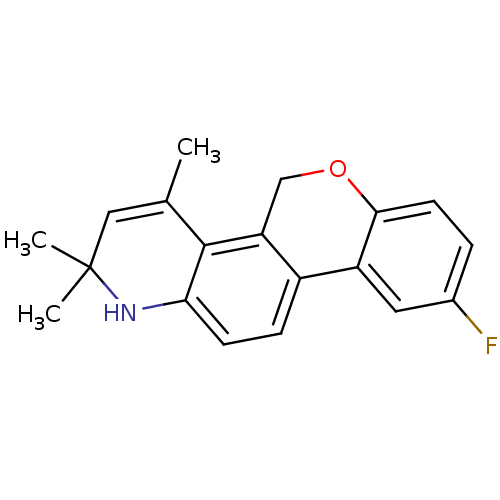 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined against hPR-A (human progesterone receptor) using progesterone radioligand in competitive binding assay | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human progesterone receptor. | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scientific Computing Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]progesterone from Progesterone receptor | J Med Chem 49: 4261-8 (2006) Article DOI: 10.1021/jm060234e BindingDB Entry DOI: 10.7270/Q2V69NB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of antagonist activity towards Androgen receptor | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonistic activity at human progesterone receptor in CV-1 cells. | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human progesterone receptor activation in T47D human breast cancer cell. | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity against hPR (human progesterone receptor) compared to that of progesterone (100%) | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 351 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Effective concentration for human progesterone receptor in T47D human breast cancer cell | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity was determined against hPR (human progesterone receptor) compared to that of progesterone (100%) | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50072715 (9-Fluoro-2,2,4-trimethyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of antagonist activity towards mineralocorticoid receptor (hMR) | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||