

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50075778 4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)-1H-imidazol-4-yl]-pyridine::4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)-3H-imidazol-4-yl]-pyridine::CHEMBL17370
SMILES: CSc1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1
InChI Key: InChIKey=ZPXPEKQMLOAPLT-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50075778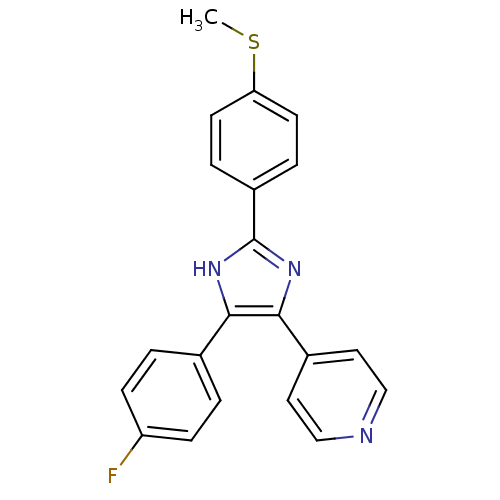 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against p38-alpha kinase | Bioorg Med Chem Lett 9: 641-6 (1999) BindingDB Entry DOI: 10.7270/Q2VD6XM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity determined by reduction in binding of 125 I-glucagon to the human glucagon receptor expressed on CHO cells in absence of Mg+2 | Bioorg Med Chem Lett 9: 641-6 (1999) BindingDB Entry DOI: 10.7270/Q2VD6XM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human glucagon receptor in absence of Mg2+ expressed as IC50 | Bioorg Med Chem Lett 11: 2549-53 (2001) BindingDB Entry DOI: 10.7270/Q2FB527Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase p38 (Homo sapiens (Human)) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human Mitogen-activated protein kinase p38 expressed as IC50 | Bioorg Med Chem Lett 11: 2549-53 (2001) BindingDB Entry DOI: 10.7270/Q2FB527Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human glucagon receptor | Citation and Details Article DOI: 10.1007/s00044-013-0801-3 BindingDB Entry DOI: 10.7270/Q20G3MM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against interleukin-1(IL-1) synthesis, using intact human monocytes | Bioorg Med Chem Lett 5: 1171-1176 (1995) Article DOI: 10.1016/0960-894X(95)00189-Z BindingDB Entry DOI: 10.7270/Q28S4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50075778 (4-[5-(4-Fluoro-phenyl)-2-(4-methylsulfanyl-phenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase enzyme from RBL-1 cells | Bioorg Med Chem Lett 5: 1171-1176 (1995) Article DOI: 10.1016/0960-894X(95)00189-Z BindingDB Entry DOI: 10.7270/Q28S4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||