

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50075971 CHEMBL3415780
SMILES: OC(=O)C([O-])=O.COc1cccc(COc2cccc(c2)C(=C)C[NH+](C)CC#C)c1
InChI Key: InChIKey=BHKOUXWCPSTZAK-UHFFFAOYSA-O
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50075971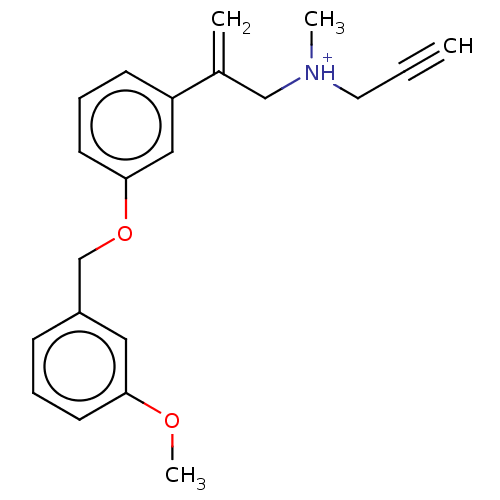 (CHEMBL3415780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B expressed in Sf9 cells using 5-phenylacetaldehyde substrate assessed as hydrogen peroxide production after 1 hr... | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50075971 (CHEMBL3415780) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in Sf9 cells using 5-hydroxytryptamine substrate assessed as hydrogen peroxide production after 1 hr ... | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine oxidase (Rattus norvegicus (rat)) | BDBM50075971 (CHEMBL3415780) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-A in nuclei-free homogenates using [14C]hydroxytryptamine substrate after 20 mins by liquid scintillation counting | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoamine Oxidase Type B (MAO-B) (Rattus norvegicus (rat)) | BDBM50075971 (CHEMBL3415780) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
A* STAR (Agency of Science, Technology and Research) Curated by ChEMBL | Assay Description Inhibition of rat brain MAO-B in nuclei-free homogenates using [14C]phenylacetaldehyde substrate after 20 mins by liquid scintillation counting | J Med Chem 58: 1400-19 (2015) Article DOI: 10.1021/jm501722s BindingDB Entry DOI: 10.7270/Q2V989R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||