

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50087657 1,2-di[4-(4-hexahydro-1-pyridinyl-1-pyridiniumylmethyl)phenyl]ethane dibromide::CHEMBL163319
SMILES: C(Cc1ccc(Cn2ccc(cc2)=[N+]2CCCCC2)cc1)c1ccc(Cn2ccc(cc2)=[N+]2CCCCC2)cc1
InChI Key: InChIKey=WKYZKQDMHOGQOV-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choline kinase alpha (Homo sapiens (Human)) | BDBM50087657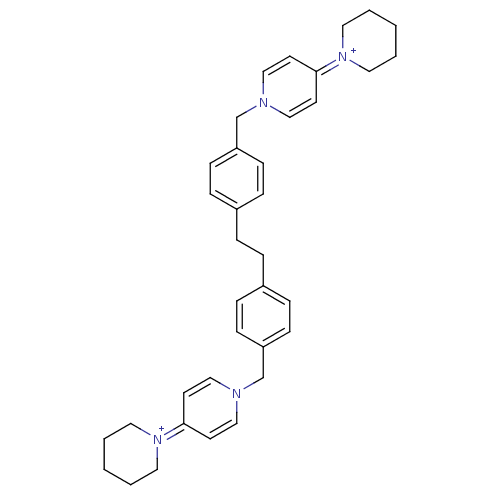 (1,2-di[4-(4-hexahydro-1-pyridinyl-1-pyridiniumylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Granada Curated by ChEMBL | Assay Description Compound was tested ex vivo for inhibition against purified choline kinase obtained from yeast | Bioorg Med Chem Lett 10: 767-70 (2000) BindingDB Entry DOI: 10.7270/Q28C9VGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50087657 (1,2-di[4-(4-hexahydro-1-pyridinyl-1-pyridiniumylme...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of equine BChE after 20 mins using butyrylthiocholine iodide as a substrate by Ellman's assay | J Med Chem 54: 2627-45 (2011) Article DOI: 10.1021/jm101299d BindingDB Entry DOI: 10.7270/Q2SQ90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50087657 (1,2-di[4-(4-hexahydro-1-pyridinyl-1-pyridiniumylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of bovine AChE after 20 mins using acetylthiocholine iodide as a substrate by Ellman's assay | J Med Chem 54: 2627-45 (2011) Article DOI: 10.1021/jm101299d BindingDB Entry DOI: 10.7270/Q2SQ90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||