

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50103347 (S)-4-((5-((2-oxo-1-(pyridin-4-yl)pyrrolidin-3-ylamino)methyl)-1H-imidazol-1-yl)methyl)benzonitrile::4-{5-[(2-Oxo-1-pyridin-4-yl-pyrrolidin-3-ylamino)-methyl]-imidazol-1-ylmethyl}-benzonitrile::CHEMBL99371
SMILES: O=C1[C@H](CCN1c1ccncc1)NCc1cncn1Cc1ccc(cc1)C#N
InChI Key: InChIKey=KDDZVJQCAMDQTB-FQEVSTJZSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl transferase type I (Homo sapiens (Human)) | BDBM50103347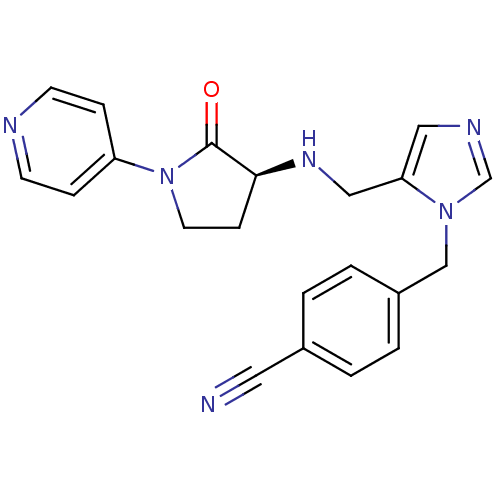 ((S)-4-((5-((2-oxo-1-(pyridin-4-yl)pyrrolidin-3-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration of geranylgeranyl-protein transferase type I | J Med Chem 44: 2933-49 (2001) BindingDB Entry DOI: 10.7270/Q2FF3RNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50103347 ((S)-4-((5-((2-oxo-1-(pyridin-4-yl)pyrrolidin-3-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG channel | J Med Chem 52: 4266-76 (2009) Article DOI: 10.1021/jm900002x BindingDB Entry DOI: 10.7270/Q2MK6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Farnesyltransferase (PFT) Chain B (Homo sapiens (Human)) | BDBM50103347 ((S)-4-((5-((2-oxo-1-(pyridin-4-yl)pyrrolidin-3-yla...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Concentration required to displace 50% of a potent radiolabeled FTI from farnesyl transferase in cultured H-ras-transformed Rat1 cells | J Med Chem 44: 2933-49 (2001) BindingDB Entry DOI: 10.7270/Q2FF3RNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Farnesyltransferase (PFT) Chain B (Homo sapiens (Human)) | BDBM50103347 ((S)-4-((5-((2-oxo-1-(pyridin-4-yl)pyrrolidin-3-yla...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of farnesyl transferase using purified recombinant human enzyme | J Med Chem 44: 2933-49 (2001) BindingDB Entry DOI: 10.7270/Q2FF3RNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||